Drug Catalog - Product Detail
CEFPROZIL TB 500MG 50
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0404-01 | LUPIN PHARMACEUTICALS | 50 | 500MG | TABLET |
PACKAGE FILES


Generic Name
CEFPROZIL
Substance Name
CEFPROZIL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA065276
Description
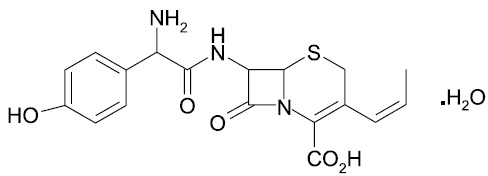
DESCRIPTION Cefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic. Cefprozil is a cis and trans isomeric mixture (≥90% cis). The chemical name for the monohydrate is (6 R ,7 R )-7-[( R )-2-Amino-2-( p -hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylic acid monohydrate, and the structural formula is: Cefprozil is a white to yellowish powder with a molecular formula for the monohydrate of C 18 H 19 N 3 O 5 S.H 2 O and a molecular weight of 407.45. Cefprozil tablets are intended for oral administration. Cefprozil tablets contain cefprozil equivalent to 250 mg or 500 mg of anhydrous cefprozil. In addition, each tablet contains the following inactive ingredients: magnesium stearate, methylcellulose, microcrystalline cellulose, sodium starch glycolate and titanium dioxide. The 250 mg tablet also contains FD & C Yellow No. 6 Aluminum Lake. Cefprozil USP
How Supplied
HOW SUPPLIED Cefprozil tablets USP, 250 mg and 500 mg are available as follows: Each light orange, film-coated, oval tablet debossed with 'LUPIN' on one side and '250' on the other side, contains the equivalent of 250 mg anhydrous cefprozil. Bottles of 100 Tablets NDC 68180-403-01 Each white, film-coated, oval tablet debossed with 'LUPIN' on one side and '500' on the other side, contains the equivalent of 500 mg anhydrous cefprozil. Bottles of 50 Tablets NDC 68180-404-01 Bottles of 100 Tablets NDC 68180-404-02 Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Cefprozil tablets are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below: Upper Respiratory Tract: Pharyngitis/Tonsillitis: Caused by Streptococcus pyogenes . NOTE: The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin given by the intramuscular route. Cefprozil is generally effective in the eradication of Streptococcus pyogenes from the nasopharynx; however, substantial data establishing the efficacy of cefprozil in the subsequent prevention of rheumatic fever are not available at present. Otitis Media: Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains). (See CLINICAL STUDIES .) NOTE: In the treatment of otitis media due to ß-lactamase producing organisms, cefprozil had bacteriologic eradication rates somewhat lower than those observed with a product containing a specific ß-lactamase inhibitor. In considering the use of cefprozil, lower overall eradication rates should be balanced against the susceptibility patterns of the common microbes in a given geographic area and the increased potential for toxicity with products containing ß-lactamase inhibitors. Acute Sinusitis: Caused by Streptococcus pneumoniae , Haemophilus influenzae (including ß-lactamase producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains). Lower Respiratory Tract: Acute Bacterial Exacerbation of Chronic Bronchitis: Caused by Streptococcus pneumoniae, Haemophilus influenzae (including ß-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including ß-lactamase-producing strains). Skin and Skin Structure: Uncomplicated Skin and Skin-Structure Infections: Caused by Staphylococcus aureus (including penicillinase-producing strains) and Streptococcus pyogenes . Abscesses usually require surgical drainage. To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil tablets and other antibacterial drugs, cefprozil tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Cefprozil tablets are administered orally. Population / Infection Dosage ( mg ) Duration ( days ) a In the treatment of infections due to Streptococcus pyogenes, cefprozil tablets should be administered for at least 10 days. b Not to exceed recommended adult doses. ADULTS (13 years and older) UPPER RESPIRATORY TRACT Pharyngitis/Tonsillitis 500 q24h 10 a Acute Sinusitis 250 q12h or 10 (For moderate to severe infections, the higher dose should be used) 500 q12h LOWER RESPIRATORY TRACT Acute Bacterial Exacerbation of Chronic Bronchitis 500 q12h 10 SKIN AND SKIN STRUCTURE Uncomplicated Skin and Skin Structure Infections 250 q12h or 10 500 q24h or 500 q12h CHILDREN (2 years to 12 years) UPPER RESPIRATORY TRACT b Pharyngitis/Tonsillitis 7.5 mg/kg q12h 10 a SKIN AND SKIN STRUCTURE Uncomplicated Skin and Skin Structure Infections 20 mg/kg q24h 10 INFANTS & CHILDREN (6 months to 12 years) UPPER RESPIRATORY TRACT b Otitis Media 15 mg/kg q12h 10 (See INDICATIONS AND USAGE and CLINICAL STUDIES ) Acute Sinusitis 7.5 mg/kg q12h or 10 (For moderate to severe infections, the higher dose should be used) 15 mg/kg q12h Renal Impairment: Cefprozil may be administered to patients with impaired renal function. The following dosage schedule should be used. Creatinine Clearance ( mL / min ) Dosage ( mg ) Dosing Interval 30 to 120 standard standard 0 to 29 Cefprozil is in part removed by hemodialysis; therefore, cefprozil should be administered after the completion of hemodialysis. 50% of standard standard Hepatic Impairment: No dosage adjustment is necessary for patients with impaired hepatic function.
