Drug Catalog - Product Detail
CEFPODOXIME PROXETIL TB 200MG 20
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00781-5439-20 | SANDOZ | 20 | 200MG | TABLET |
PACKAGE FILES



Generic Name
CEFPODOXIME PROXETIL
Substance Name
CEFPODOXIME PROXETIL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA065462
Description
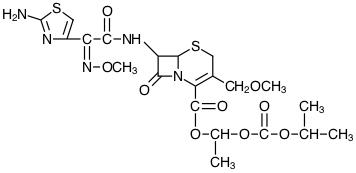
DESCRIPTION Cefpodoxime proxetil is an orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class. The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R,7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z)methoxyimino} acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene-2-carboxylate. Its structural formula is represented below: Molecular Formula: C 21 H 27 N 5 O 9 S 2 Molecular Weight: 557.6 Cefpodoxime proxetil is a prodrug; its active metabolite is cefpodoxime. All doses of cefpodoxime proxetil in this insert are expressed in terms of the active cefpodoxime moiety. The drug is supplied as film-coated tablets. Cefpodoxime proxetil tablets, USP contain cefpodoxime proxetil equivalent to 100 mg or 200 mg of cefpodoxime activity. Each film-coated tablet contains the following inactive ingredients: carboxymethylcellulose calcium, crospovidone, FD&C Yellow No. 6, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, sodium lauryl sulfate, talc and titanium dioxide. chemical-structure
How Supplied
HOW SUPPLIED Cefpodoxime Proxetil Tablets, USP are available in the following strengths (cefpodoxime equivalent), colors, and sizes: 100 mg, (light orange, film-coated, elliptical, embossed with SZ 438) Bottles of 20 NDC 0781-5438-20 Bottles of 100 NDC 0781-5438-01 200 mg, (light orange, film-coated, oblong, embossed with SZ 439) Bottles of 20 NDC 0781-5439-20 Bottles of 100 NDC 0781-5439-01 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Replace cap securely after each opening. KEEP OUT OF THE REACH OF CHILDREN
Indications & Usage
INDICATIONS AND USAGE Cefpodoxime proxetil is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below. Recommended dosages, durations of therapy, and applicable patient populations vary among these infections. Please see DOSAGE AND ADMINISTRATION for specific recommendations. Acute otitis media caused by Streptococcus pneumoniae (excluding penicillin-resistant strains), Streptococcus pyogenes, Haemophilus influenzae (including beta-lactamase-producing strains), or Moraxella (Branhamella) catarrhalis (including beta-lactamase-producing strains). Pharyngitis and/or tonsillitis caused by Streptococcus pyogenes . NOTE: Only penicillin by the intramuscular route of administration has been shown to be effective in the prophylaxis of rheumatic fever. Cefpodoxime proxetil is generally effective in the eradication of streptococci from the oropharynx. However, data establishing the efficacy of cefpodoxime proxetil for the prophylaxis of subsequent rheumatic fever are not available. Community-acquired pneumonia caused by S.pneumoniae or H.influenzae (including beta-lactamase-producing strains). Acute bacterial exacerbation of chronic bronchitis caused by S.pneumoniae, H.influenzae (non-beta-lactamase-producing strains only), or M.catarrhalis . Data are insufficient at this time to establish efficacy in patients with acute bacterial exacerbations of chronic bronchitis caused by beta-lactamase-producing strains of H.influenzae . Acute, uncomplicated urethral and cervical gonorrhea caused by Neisseria gonorrhoeae (including penicillinase-producing strains). Acute, uncomplicated ano-rectal infections in women due to Neisseria gonorrhoeae (including penicillinase-producing strains). NOTE: The efficacy of cefpodoxime in treating male patients with rectal infections caused by N.gonorrhoeae has not been established. Data do not support the use of cefpodoxime proxetil in the treatment of pharyngeal infections due to N.gonorrhoeae in men or women. Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (including penicillinase-producing strains) or Streptococcus pyogenes . Abscesses should be surgically drained as clinically indicated. NOTE: In clinical trials, successful treatment of uncomplicated skin and skin structure infections was dose-related. The effective therapeutic dose for skin infections was higher than those used in other recommended indications (see DOSAGE AND ADMINISTRATION ). Acute maxillary sinusitis caused by Haemophilus influenzae (including beta-lactamase-producing strains), Streptococcus pneumoniae, and Moraxella catarrhalis . Uncomplicated urinary tract infections (cystitis) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis , or Staphylococcus saprophyticus . NOTE: In considering the use of cefpodoxime proxetil in the treatment of cystitis, cefpodoxime proxetil’s lower bacterial eradication rates should be weighed against the increased eradication rates and different safety profiles of some other classes of approved agents (see CLINICAL STUDIES section). Appropriate specimens for bacteriological examination should be obtained in order to isolate and identify causative organisms and to determine their susceptibility to cefpodoxime. Therapy may be instituted while awaiting the results of these studies. Once these results become available, antimicrobial therapy should be adjusted accordingly. To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefpodoxime proxetil tablets and other antibacterial drugs, Cefpodoxime proxetil tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION (See INDICATIONS AND USAGE for indicated pathogens.) Film-coated Tablets Cefpodoxime proxetil tablets, USP should be administered orally with food to enhance absorption (see CLINICAL PHARMACOLOGY ). The recommended dosages, durations of treatment, and applicable patient population are as described in the following chart: Adults and Adolescents (age 12 years and older) Type of Infection Total Daily Dose Dose Frequency Duration Pharyngitis and/or tonsillitis 200 mg 100 mg Q 12 hours 5 to 10 days Acute community-acquired pneumonia 400 mg 200 mg Q 12 hours 14 days Acute bacterial exacerbations of chronic bronchitis 400 mg 200 mg Q 12 hours 10 days Uncomplicated gonorrhea (men and women) and rectal gonococcal infections (women) 200 mg single dose Skin and skin structure 800 mg 400 mg Q 12 hours 7 to 14 days Acute maxillary sinusitis 400 mg 200 mg Q 12 hours 10 days Uncomplicated urinary tract infection 200 mg 100 mg Q 12 hours 7 days Patients with Renal Dysfunction For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis. When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function. Males: (mL/min) Weight (kg) × (140 - age) 72 × serum creatinine (mg/100 mL) Females: (mL/min) 0.85 × above value Patients with Cirrhosis Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population.
