Drug Catalog - Product Detail
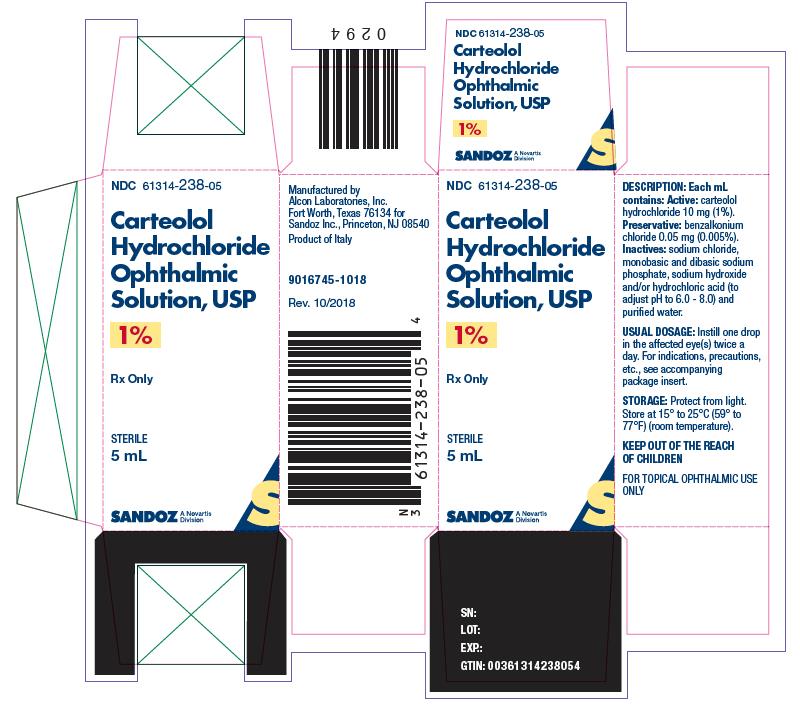
CARTEOLOL HCL OPHTH. SOL. SOL 0.01 5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61314-0238-05 | SANDOZ | 5 | 1% | NA |
PACKAGE FILES
Generic Name
CARTEOLOL HYDROCHLORIDE
Substance Name
CARTEOLOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
ANDA075476
Description
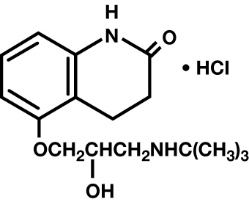
DESCRIPTION Carteolol Hydrochloride Ophthalmic Solution USP, 1% is a nonselective beta-adrenoceptor blocking agent for ophthalmic use. The chemical name for carteolol hydrochloride is (±)-5-[3-[(1,1-dimethylethyl) amino]-2-hydroxypropoxy]-3,4-dihydro-2(1H)-quinolinone monohydrochloride. The structural formula is as follows: C 16 H 24 N 2 O 3 •HCI Mol. Wt. 328.84 Each mL of sterile solution contains Active: carteolol hydrochloride 10 mg (1%). Preservative: benzalkonium chloride 0.05 mg (0.005%). Inactives: sodium chloride, monobasic and dibasic sodium phosphate, sodium hydroxide and/or hydrochloric acid (to adjust pH to 6.0 - 8.0) and purified water. chemical
How Supplied
HOW SUPPLIED Carteolol Hydrochloride Ophthalmic Solution USP, 1% is supplied as a sterile ophthalmic solution in plastic dispenser bottles of 5 mL (NDC 61314-238-05), 10 mL (NDC 61314-238-10) and 15 mL (NDC 61314-238-15). Store at 15° to 25°C (59° to 77°F) (room temperature) and protect from light.
Indications & Usage
INDICATIONS AND USAGE Carteolol Hydrochloride Ophthalmic Solution 1% has been shown to be effective in lowering intraocular pressure and may be used in patients with chronic open-angle glaucoma and intraocular hypertension. It may be used alone or in combination with other intraocular pressure lowering medications.
Dosage and Administration
DOSAGE AND ADMINISTRATION The usual dose is one drop of Carteolol Hydrochloride Ophthalmic Solution 1% in the affected eye(s) twice a day. If the patient's IOP is not at a satisfactory level on this regimen, concomitant therapy with pilocarpine and other miotics, and/or epinephrine or dipivefrin, and/or systemically administered carbonic anhydrase inhibitors, such as acetazolamide, can be instituted.
