Drug Catalog - Product Detail
CALCIUM ACETATE (PHOSPHATE BINDER) TAB 667 MG 200 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 23155-0621-02 | AVET PHARMACEUTICALS | 200 | 667MG | TABLET |
PACKAGE FILES


Generic Name
CALCIUM ACETATE
Substance Name
CALCIUM ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202885
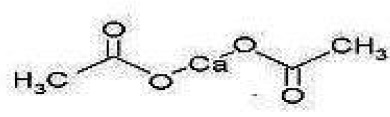
Description
11 DESCRIPTION Calcium acetate tablet acts as a phosphate binder. Its chemical name is calcium acetate. Its molecular formula is C 4 H 6 CaO 4 , and its molecular weight is 158.17. Its structural formula is: Each calcium acetate tablet contains 667 mg of calcium acetate, (anhydrous; Ca (CH 3 COO) 2 ; MW = 158.17 grams) equal to 169 mg (8.45 mEq) calcium. In addition, each tablet contains following inactive ingredients: crospovidone, magnesium stearate and sodium lauryl sulfate. Calcium acetate tablets are administered orally for the control of hyperphosphatemia in end stage renal failure. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Each Calcium Acetate Tablet USP, intended for oral administration, is white, round tablet, Debossed EP 114 on one side and plain on the reverse side. Each calcium acetate tablet contains 667 mg of calcium acetate (anhydrous Ca(CH 3 COO) 2 ; MW=158.17 grams) equal to 169 mg (8.45 mEq) calcium and are supplied as follow: Bottles of 200: NDC 23155-621-02 STORAGE: Store at 20° to 25°C (68° to 77°F) excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS & USAGE Calcium acetate tablet is a phosphate binder indicated to reduce serum phosphorus in patients with end stage renal disease (ESRD). Calcium acetate tablet is a phosphate binder indicated for the reduction of serum phosphorus in patients with end stage renal disease. ( 1 )
Dosage and Administration
2 DOSAGE & ADMINISTRATION The recommended initial dose of calcium acetate tablets for the adult dialysis patient is 2 tablets with each meal. Increase the dose gradually to lower serum phosphorus levels to the target range, as long as hypercalcemia does not develop. Most patients require 3 to 4 tablets with each meal. Starting dose is 2 tablets with each meal. (2) Titrate the dose every 2 to 3 weeks until acceptable serum phosphorus level is reached. Most patients require 3 to 4 tablets with each meal. (2)
