Drug Catalog - Product Detail
BUTALBITAL/ACETAMINOPHEN & CAFFEINE TABS. TB 50/325/40MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00603-2544-28 | ENDO USA | 500 | 50-325-40MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
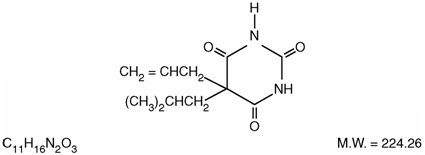
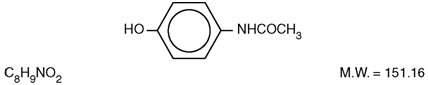
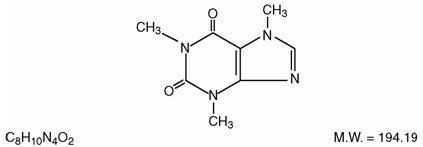
DESCRIPTION Butalbital, Acetaminophen and Caffeine Tablets, USP are supplied in tablet form for oral administration. Each tablet contains the following active ingredients: butalbital, USP ...................................................................................... 50 mg acetaminophen, USP ........................................................................... 325 mg caffeine, USP ........................................................................................ 40 mg Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, crospovidone, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, and stearic acid. Butalbital (5-allyl-5-isobutylbarbituric acid), is a short to intermediate-acting barbiturate. It has the following structural formula: Acetaminophen (4'-hydroxyacetanilide), is a non-opiate, non-salicylate analgesic and antipyretic. It has the following structural formula. Caffeine (1,3,7-trimethylxanthine), is a central nervous system stimulant. It has the following structural formula: This is an image of the structural formula of Butalbital. This is an image of the structural formula of Acetaminophen. This is an image of the structural formula of Caffeine.
How Supplied
HOW SUPPLIED Repackaged by Aphena Pharma Solutions - TN. See Repackaging Information for available configurations. Butalbital, Acetaminophen and Caffeine Tablets, USP Containing 50 mg butalbital, 325 mg acetaminophen and 40 mg caffeine. Available as white, round shaped tablets, debossed "2355" on one side, and debossed "V" on the reverse side. Bottles of 60: NDC 0603-2544-20 Bottles of 90: NDC 0603-2544-02 Bottles of 100: NDC 0603-2544-21 Bottles of 500: NDC 0603-2544-28 Bottles of 1000: NDC 0603-2544-32 Store and Dispense Store at 20°-25°C (68°-77°F) with excursions permitted between 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]; dispense in a tight, light-resistant container as defined in the USP.
Indications & Usage
INDICATIONS AND USAGE Butalbital, acetaminophen and caffeine tablets are indicated for the relief of the symptom complex of tension (or muscle contraction) headache. Evidence supporting the efficacy and safety of this combination product in the treatment of multiple recurrent headaches is unavailable. Caution in this regard is required because butalbital is habit-forming and potentially abusable.
Dosage and Administration
DOSAGE AND ADMINISTRATION One or 2 tablets every 4 hours as needed. Total daily dosage should not exceed 6 tablets. Extended and repeated use of this product is not recommended because of the potential for physical dependence.
