Drug Catalog - Product Detail
BUPROPION HCL ER (XL) TB 150MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-6811-10 | AMNEAL PHARMACEUTICALS | 90 | 150MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
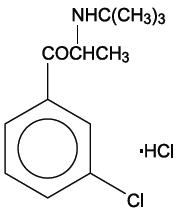
11 DESCRIPTION Bupropion hydrochloride extended-release tablets (XL), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-1-(3-chorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propanone hydrochloride. The molecular weight is 276.2. The molecular formula is C 13 H 18 ClNO∙HCl. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. The structural formula is: Bupropion hydrochloride extended-release tablets (XL) are supplied for oral administration as 150-mg, yellow extended-release tablets. Each tablet contains the labeled amount of bupropion hydrochloride and the inactive ingredients: colloidal silicon dioxide, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate and microcrystalline cellulose. The filmcoating material contains FD&C red #40, FD&C yellow #5, hypromellose type 2910/3 cP, 6 cP and 50 cP, macrogol, polydextrose, titanium dioxide and triacetin. Bupropion hydrochloride extended-release tablets (XL) meet USP Dissolution Test 6. The insoluble shell of the extended-release tablet may remain intact during gastrointestinal transit and is eliminated in the feces. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Bupropion hydrochloride extended-release tablets (XL), 150 mg of bupropion hydrochloride, are yellow, oval, film-coated tablets, debossed with "681" on one side and plain on the other side. Bottles of 30 NDC 0115-6811-08 Bottles of 90 NDC 0115-6811-10 Bottles of 500 NDC 0115-6811-02 Dispense in a tightly-closed, light-resistant container (USP)." Store at 20°C to 25°C (68°F to 77°F); excursion permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Bupropion hydrochloride extended-release tablets (XL) may have an odor.
Indications & Usage
1 INDICATIONS AND USAGE Bupropion hydrochloride extended-release tablets (XL) are an aminoketone antidepressant, indicated for the treatment of major depressive disorder (MDD) and prevention of seasonal affective disorder (SAD). Periodically reevaluate long-term usefulness for the individual patient. ( 1 ) 1.1 Major Depressive Disorder Bupropion hydrochloride extended-release tablets (XL) are indicated for the treatment of major depressive disorder (MDD), as defined by the Diagnostic and Statistical Manual (DSM). The efficacy of the immediate-release formulation of bupropion was established in two 4-week controlled inpatient trials and one 6-week controlled outpatient trial of adult patients with MDD. The efficacy of the sustained-release formulation of bupropion in the maintenance treatment of MDD was established in a long-term (up to 44 weeks), placebo-controlled trial in patients who had responded to bupropion in an 8-week study of acute treatment [see Clinical Studies (14.1) ]. 1.2 Seasonal Affective Disorder Bupropion hydrochloride extended-release tablets (XL) are indicated for the prevention of seasonal major depressive episodes in patients with a diagnosis of seasonal affective disorder (SAD). The efficacy of bupropion hydrochloride extended-release tablets in the prevention of seasonal major depressive episodes was established in 3 placebo-controlled trials in adult outpatients with a history of MDD with an autumn-winter seasonal pattern as defined in the DSM [see Clinical Studies (14.2) ].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION General: Increase dose gradually to reduce seizure risk. ( 2.1 , 5.3 ) Periodically reassess the dose and need for maintenance treatment. ( 2.2 ) Major Depressive Disorder Starting dose: 150 mg/day once daily. Usual target dose: 300 mg once daily ( 2.2 ) After 4 days, may increase the dose to 300 mg once daily. ( 2.2 ) Seasonal Affective Disorder Initiative treatment in the autumn prior to onset of seasonal depressive symptoms. ( 2.3 ) Starting dose: 150 mg once daily. Usual target dose: 300 mg once daily. ( 2.3 ) After one week, may increase the dose to 300 mg once daily. ( 2.3 ) Continue treatment through the winter season. ( 2.3 ) Hepatic Impairment Moderate to severe hepatic impairment: 150 mg every other day ( 2.6 ) Mild hepatic impairment: Consider reducing the dose and/or frequency of dosing. ( 2.6 , 8.7 ) Renal Impairment Consider reducing the dose and/or frequency of dosing. ( 2.7 , 8.6 ) 2.1 General Instructions for Use To minimize the risk of seizure, increase the dose gradually [see Warnings and Precautions (5.3) ]. Bupropion hydrochloride extended-release tablets (XL) should be swallowed whole and not crushed, divided, or chewed. Bupropion hydrochloride extended-release tablets (XL) should be administered in the morning and may be taken with or without food. 2.2 Dosage for Major Depressive Disorder (MDD) The recommended starting dose for MDD is 150 mg once daily in the morning. After 4 days of dosing, the dose may be increased to the target dose of 300 mg once daily in the morning. It is generally agreed that acute episodes of depression require several months or longer of antidepressant treatment beyond the response in the acute episode. It is unknown whether the bupropion hydrochloride extended-release tablets (XL) dose needed for maintenance treatment is identical to the dose that provided an initial response. Periodically reassess the need for maintenance treatment and the appropriate dose for such treatment. 2.3 Dosage for Seasonal Affective Disorder (SAD) The recommended starting dose for SAD is 150 mg once daily. After 7 days of dosing, the dose may be increased to the target dose of 300 mg once daily in the morning. Doses above 300 mg of bupropion HCl extended-release were not assessed in the SAD trials. For the prevention of seasonal MDD episodes associated with SAD, initiate bupropion hydrochloride extended-release tablets (XL) in the autumn, prior to the onset of depressive symptoms. Continue treatment through the winter season. Taper and discontinue bupropion hydrochloride extended-release tablets (XL ) in early spring. For patients treated with 300 mg per day, decrease the dose to 150 mg once daily before discontinuing bupropion hydrochloride extended-release tablets (XL). Individualize the timing of initiation and duration of treatment should be individualized, based on the patient's historical pattern of seasonal MDD episodes. 2.4 Switching Patients from WELLBUTRIN Tablets or from WELLBUTRIN SR Sustained-Release Tablets When switching patients from WELLBUTRIN Tablets to bupropion hydrochloride extended-release tablets (XL) or from WELLBUTRIN SR Sustained-Release Tablets to bupropion hydrochloride extended-release tablets (XL), give the same total daily dose when possible. 2.5 To Discontinue Bupropion Hydrochloride Extended-Release Tablets (XL), Taper the Dose When discontinuing treatment in patients treated with bupropion hydrochloride extended-release tablets (XL) 300 mg once daily, decrease the dose to 150 mg once daily prior to discontinuation. 2.6 Dosage Adjustment in Patients with Hepatic Impairment In patients with moderate to severe hepatic impairment (Child-Pugh score: 7 to 15), the maximum dose is 150 mg every other day. In patients with mild hepatic impairment (Child-Pugh score: 5 to 6), consider reducing the dose and/or frequency of dosing [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3) ]. 2.7 Dose Adjustment in Patients with Renal Impairment Consider reducing the dose and/or frequency of WELLBUTRIN in patients with renal impairment (Glomerular Filtration Rate <90 mL/min) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ]. 2.8 Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Antidepressant At least 14 days should elapse between discontinuation of an MAOI intended to treat depression and initiation of therapy with bupropion hydrochloride extended-release tablets (XL). Conversely, at least 14 days should be allowed after stopping bupropion hydrochloride extended-release tablets (XL) before starting an MAOI antidepressant [see Contraindications (4) and Drug Interactions (7.6) ]. 2.9 Use of Bupropion Hydrochloride Extended-Release Tablets (XL) with Reversible MAOIs Such as Linezolid or Methylene Blue Do not start bupropion hydrochloride extended-release tablets (XL) in a patient who is being treated with a reversible MAOI such as linezolid or intravenous methylene blue. Drug interactions can increase risk of hypertensive reactions. In a patient who requires more urgent treatment of a psychiatric condition, non-pharmacological interventions, including hospitalization, should be considered [see Contraindications (4) ]. In some cases, a patient already receiving therapy with bupropion hydrochloride extended-release tablets (XL) may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of hypertensive reactions in a particular patient, bupropion hydrochloride extended-release tablets (XL) should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with bupropion hydrochloride extended-release tablets (XL) may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue. The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with bupropion hydrochloride extended-release tablets (XL) is unclear. The clinician should, nevertheless, be aware of the possibility of a drug interaction with such use [see Contraindications (4) and Drug Interactions (7.6) ].
