Drug Catalog - Product Detail
BUPROPION HCL ER SR 100MG TB 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43547-0288-10 | SOLCO HEALTHCARE | 100 | 100MG | TABLET |
PACKAGE FILES



Generic Name
BUPROPION HYDROCHLORIDE
Substance Name
BUPROPION HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202304
Description
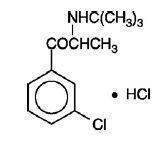
11 DESCRIPTION Bupropion hydrochloride extended-release tablets USP (SR), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propanone hydrochloride. The molecular weight is 276.2. The molecular formula is C 13 H 18 ClNO ∙ HCl. Bupropion hydrochloride, USP powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. The structural formula is: Bupropion hydrochloride extended-release tablets USP (SR) is supplied for oral administration as 100-mg (blue), 150-mg (purple), and 200-mg (pink), film-coated, sustained-release tablets. Each tablet contains the labeled amount of bupropion hydrochloride, USP and the inactive ingredients: cysteine hydrochloride, hypromellose, magnesium stearate, microcrystalline cellulose, talc and titanium dioxide. In addition, the 100-mg tablet contains FD&C Blue No. 2 Lake, the 150-mg tablet contains FD&C Blue No. 2 Lake and FD&C Red No. 40 Lake, and the 200-mg tablet contains Iron Oxide Red and Ferrosoferric Oxide. Meet USP Dissolution Test 7. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Bupropion hydrochloride extended-release tablets USP (SR), 100 mg of bupropion hydrochloride, USP are blue, round, biconvex, film-coated tablets debossed with “S” on one side and “522” on the other. Bottles of 60 NDC # 43547-288-06 Bottles of 90 NDC # 43547-288-09 Bottles of 100 NDC # 43547-288-10 Bottles of 500 NDC # 43547-288-50 Bupropion hydrochloride, USP extended-release tablets USP (SR), 150 mg of bupropion hydrochloride, USP are purple, round, biconvex, film-coated tablets debossed with “S” on one side and “525” on the other. Bottles of 60 NDC # 43547-289-06 Bottles of 90 NDC # 43547-289-09 Bottles of 100 NDC # 43547-289-10 Bottles of 250 NDC # 43547-289-25 Bottles of 500 NDC # 43547-289-50 Bupropion hydrochloride extended-release tablets USP (SR), 200 mg of bupropion hydrochloride, USP are pink, round, biconvex, film-coated tablets debossed with “S” on one side and “527” on the other. Bottles of 60 NDC # 43547-290-06 Bottles of 90 NDC # 43547-290-09 Bottles of 100 NDC # 43547-290-10 Bottles of 500 NDC # 43547-290-50 Store at room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Protect from light and moisture.
Indications & Usage
1 INDICATIONS AND USAGE Bupropion hydrochloride extended-release (SR) tablets are indicated for the treatment of major depressive disorder (MDD), as defined by the Diagnostic and Statistical Manual (DSM). The efficacy of bupropion in the treatment of a major depressive episode was established in two 4-week controlled inpatient trials and one 6-week controlled outpatient trial of adult subjects with MDD [see Clinical Studies (14) ] . The efficacy of bupropion hydrochloride extended-release (SR) tablets in maintaining an antidepressant response for up to 44 weeks following 8 weeks of acute treatment was demonstrated in a placebo-controlled trial [see Clinical Studies (14) ] . Bupropion hydrochloride extended-release (SR) tablets are an aminoketone antidepressant, indicated for the treatment of major depressive disorder (MDD). ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Starting dose: 150 mg/day. ( 2.1 ) • General: Increase dose gradually to reduce seizure risk. ( 2.1 , 5.3 ) • After 3 days, may increase the dose to 300 mg/day, given as 150 mg twice daily at an interval of at least 8 hours. ( 2.1 ) • Usual target dose: 300 mg/day as 150 mg twice daily. ( 2.1 ) • Maximum dose: 400 mg/day, given as 200 mg twice daily, for patients not responding to 300 mg/day. ( 2.1 ) • Periodically reassess the dose and need for maintenance treatment. ( 2.1 ) • Moderate to severe hepatic impairment: 100 mg daily or 150 mg every other day. ( 2.2 , 8.7 ) • Mild hepatic impairment: Consider reducing the dose and/or frequency of dosing. ( 2.2 , 8.7 ) • Renal impairment: Consider reducing the dose and/or frequency. ( 2.3 , 8.6 ) 2.1 General Instructions for Use To minimize the risk of seizure, increase the dose gradually [see Warnings and Precautions (5.3) ] . Bupropion hydrochloride extended-release (SR) tablets should be swallowed whole and not crushed, divided, or chewed. Bupropion hydrochloride extended-release (SR) tablets may be taken with or without food. The usual adult target dose for bupropion hydrochloride extended-release (SR) tablets is 300 mg/day, given as 150 mg twice daily. Initiate dosing with 150 mg/day given as a single daily dose in the morning. After 3 days of dosing, the dose may be increased to the 300-mg/day target dose, given as 150 mg twice daily. There should be an interval of at least 8 hours between successive doses. A maximum of 400 mg/day, given as 200 mg twice daily, may be considered for patients in whom no clinical improvement is noted after several weeks of treatment at 300 mg/day. To avoid high peak concentrations of bupropion and/or its metabolites, do not exceed 200 mg in any single dose. It is generally agreed that acute episodes of depression require several months or longer of antidepressant drug treatment beyond the response in the acute episode. It is unknown whether the dose of bupropion hydrochloride extended-release (SR) tablets needed for maintenance treatment is identical to the dose that provided an initial response. Periodically reassess the need for maintenance treatment and the appropriate dose for such treatment. 2.2 Dose Adjustment in Patients with Hepatic Impairment In patients with moderate to severe hepatic impairment (Child-Pugh score: 7 to 15), the maximum dose of bupropion hydrochloride extended-release (SR) tablets is 100 mg/day or 150 mg every other day. In patients with mild hepatic impairment (Child-Pugh score: 5 to 6), consider reducing the dose and/or frequency of dosing [see Use in Specific Populations (8.7) , Clinical Pharmacology (12.3) ] . 2.3 Dose Adjustment in Patients with Renal Impairment Consider reducing the dose and/or frequency of bupropion hydrochloride extended-release (SR) tablets in patients with renal impairment (Glomerular Filtration Rate [GFR] less than 90 mL/min) [see Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ] . 2.4 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant At least 14 days should elapse between discontinuation of an MAOI intended to treat depression and initiation of therapy with bupropion hydrochloride extended-release (SR) tablets. Conversely, at least 14 days should be allowed after stopping bupropion hydrochloride extended-release (SR) tablets before starting an MAOI antidepressant [see Contraindications (4) , Drug Interactions (7.6) ] . 2.5 Use of Bupropion Hydrochloride Extended-release (SR) Tablets with Reversible MAOIs Such as Linezolid or Methylene Blue Do not start bupropion hydrochloride extended-release (SR) in a patient who is being treated with a reversible MAOI such as linezolid or intravenous methylene blue. Drug interactions can increase the risk of hypertensive reactions. In a patient who requires more urgent treatment of a psychiatric condition, non-pharmacological interventions, including hospitalization, should be considered [see Contraindications (4) , Drug Interactions (7.6) ] . In some cases, a patient already receiving therapy with bupropion hydrochloride extended-release (SR) tablets may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of hypertensive reactions in a particular patient, bupropion hydrochloride extended-release (SR) tablets should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with bupropion hydrochloride extended-release (SR) tablets may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue. The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with bupropion hydrochloride extended-release (SR) tablets is unclear. The clinician should, nevertheless, be aware of the possibility of a drug interaction with such use [see Contraindications (4) , Drug Interactions (7.6) ] .
