Drug Catalog - Product Detail
Bumetanide Tab 0.5 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68382-0525-01 | ZYDUS PHARMACEUTICALS (USA) | 100 | 0.5MG | TABLET |
PACKAGE FILES


Generic Name
BUMETANIDE
Substance Name
BUMETANIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202900
Description
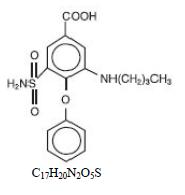
DESCRIPTION Bumetanide is a loop diuretic, available as scored tablets. Chemically, bumetanide is 3-(butylamino)-4-phenoxy-5-sulfamoylbenzoic acid. It is a practically white powder having a calculated molecular weight of 364.42, and the following structural formula: Each bumetanide tablet intended for oral administration contains 0.5 mg, 1 mg or 2 mg of bumetanide. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, povidone and talc. In addition, each 0.5 mg tablet contains D&C Yellow No. 10 aluminum lake, FD&C Blue No. 1 aluminum lake and ferric oxide black; each 1 mg tablet contains D&C Yellow No. 10 aluminum lake and ferric oxide red; each 2 mg tablet contains mg D&C Yellow No. 10 aluminum lake and ferric oxide red. Bumetanide tablet meets USP Dissolution Test 2. figure 1
How Supplied
HOW SUPPLIED Bumetanide Tablets USP, 0.5 mg are light green, round, biconvex, uncoated tablet debossed with '525' on one side separating '5' & '25' with breakline and plain on the other side and are supplied as follows: NDC 68382-525-06 in bottles of 30 tablets with child-resistance closure NDC 68382-525-16 in bottles of 90 tablets with child-resistance closure NDC 68382-525-01 in bottles of 100 tablets NDC 68382-525-05 in bottles of 500 tablets NDC 68382-525-10 in bottles of 1000 tablets Bumetanide Tablets USP, 1 mg are light yellow, round, biconvex, uncoated tablet debossed with '526' on one side separating '5' & '26' with breakline and plain on the other side and are supplied as follows: NDC 68382-526-06 in bottles of 30 tablets with child-resistance closure NDC 68382-526-16 in bottles of 90 tablets with child-resistance closure NDC 68382-526-01 in bottles of 100 tablets NDC 68382-526-05 in bottles of 500 tablets NDC 68382-526-10 in bottles of 1000 tablets Bumetanide Tablets USP, 2 mg are light orange, round, biconvex, uncoated tablet debossed with '527' on one side separating '5' & '27' with breakline and plain on the other side and are supplied as follows: NDC 68382-527-06 in bottles of 30 tablets with child-resistance closure NDC 68382-527-16 in bottles of 90 tablets with child-resistance closure NDC 68382-527-01 in bottles of 100 tablets NDC 68382-527-05 in bottles of 500 tablets NDC 68382-527-10 in bottles of 1000 tablets Storage Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container (USP). Manufactured by: Zydus Lifesciences Ltd., Baddi, India Distributed by: Zydus Pharmaceuticals ( USA) Inc. Pennington, NJ 08534 Rev.: 01/23
Indications & Usage
INDICATIONS AND USAGE Bumetanide tablets USP are indicated for the treatment of edema associated with congestive heart failure, hepatic and renal disease, including the nephrotic syndrome. Almost equal diuretic response occurs after oral and parenteral administration of bumetanide. Therefore, if impaired gastrointestinal absorption is suspected or oral administration is not practical, bumetanide should be given by the intramuscular or intravenous route. Successful treatment with bumetanide tablets USP following instances of allergic reactions to furosemide suggests a lack of cross-sensitivity. CONTRAINDICATIONS Bumetanide is contraindicated in anuria. Although bumetanide tablets can be used to induce diuresis in renal insufficiency, any marked increase in blood urea nitrogen or creatinine, or the development of oliguria during therapy of patients with progressive renal disease, is an indication for discontinuation of treatment with bumetanide tablets. Bumetanide is also contraindicated in patients in hepatic coma or in states of severe electrolyte depletion until the condition is improved or corrected. Bumetanide is contraindicated in patients hypersensitive to this drug.
Dosage and Administration
DOSAGE AND ADMINISTRATION Individualize dosage with careful monitoring of patient response. Oral Administration The usual total daily dosage of bumetanide tablet is 0.5 mg to 2 mg and in most patients is given as a single dose. If the diuretic response to an initial dose of bumetanide tablet is not adequate, in view of its rapid onset and short duration of action, a second or third dose may be given at 4 to 5 hour intervals up to a maximum daily dose of 10 mg. An intermittent dose schedule, whereby bumetanide tablets are given on alternate days or for 3 to 4 days with rest periods of 1 to 2 days in between, is recommended as the safest and most effective method for the continued control of edema. In patients with hepatic failure, keep the dosage to a minimum. Because cross-sensitivity with furosemide has rarely been observed, bumetanide can be substituted at approximately a 1:40 ratio of bumetanide in proportion to furosemide in patients allergic to furosemide. Parenteral Administration Bumetanide injection may be administered parenterally (intravenously and intramuscularly) to patients in whom gastrointestinal absorption may be impaired or in whom oral administration is not practical. Terminate parenteral treatment and institute oral treatment as soon as possible.
