Drug Catalog - Product Detail
BUMETANIDE INJ. INJECT. 0.25MG/ML 10X4ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00641-6008-10 | HIKMA PHARMACEUTICALS USA | 4 | 0.25MG/ML | SOLUTION |
PACKAGE FILES



Generic Name
BUMETANIDE
Substance Name
BUMETANIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAMUSCULAR
Application Number
ANDA079196
Description
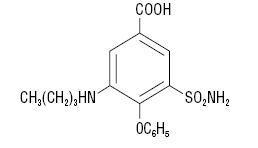
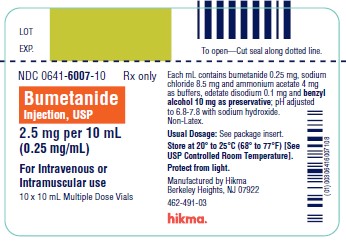
DESCRIPTION Bumetanide is a loop diuretic, available as 4 mL vials and 10 mL vials (0.25 mg/mL) for intravenous or intramuscular injection as a sterile solution. Each mL contains bumetanide 0.25 mg, sodium chloride 8.5 mg and ammonium acetate 4 mg as buffers, edetate disodium 0.1 mg and benzyl alcohol 10 mg as preservative in Water for Injection. pH adjusted to 6.8 – 7.8 with sodium hydroxide. Chemically, bumetanide is 3-(butylamino)-4-phenoxy-5-sulfamoylbenzoic acid. It is a practically white powder, slightly soluble in water, soluble in alkaline solutions, having the following structural formula: C 17 H 20 N 2 O 5 S Molecular weight: 364.42 Structural formula
How Supplied
HOW SUPPLIED Bumetanide Injection, USP, 0.25 mg/mL is a sterile, clear, colorless to slightly yellow solution supplied in amber vials as follows: 4 mL Single Dose Vial packaged in 10s (NDC 0641-6008-10) 10 mL Multiple Dose Vial packaged in 10s (NDC 0641-6007-10) This product, including the packaging components, is free of latex. Storage Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30° C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light. To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . For Product Inquiry call 1-877-845-0689. Manufactured by: Hikma Pharmaceuticals USA Inc. Berkeley Heights, NJ 07922 Revised December 2019 462-485-02
Indications & Usage
INDICATIONS AND USAGE Bumetanide Injection is indicated for the treatment of edema associated with congestive heart failure, hepatic and renal disease, including the nephrotic syndrome. Almost equal diuretic response occurs after oral and parenteral administration of bumetanide. Therefore, if impaired gastrointestinal absorption is suspected or oral administration is not practical, bumetanide should be given by the intramuscular or intravenous route. Successful treatment with bumetanide following instances of allergic reactions to furosemide suggests a lack of cross-sensitivity.
Dosage and Administration
DOSAGE AND ADMINISTRATION Dosage should be individualized with careful monitoring of patient response. Parenteral Administration Bumetanide Injection may be administered parenterally (IV or IM) to patients in whom gastrointestinal absorption may be impaired or in whom oral administration is not practical. Parenteral treatment should be terminated and oral treatment instituted as soon as possible. The usual initial dose is 0.5 to 1 mg intravenously or intramuscularly. Intravenous administration should be given over a period of 1 to 2 minutes. If the response to an initial dose is deemed insufficient, a second or third dose may be given at intervals of 2 to 3 hours, but should not exceed a daily dosage of 10 mg. Miscibility and Parenteral Solutions The compatibility tests of bumetanide injection with 5% Dextrose Injection in Water, 0.9% Sodium Chloride Injection, and Lactated Ringer’s Injection in both glass and plasticized PVC (Viaflex) containers have shown no significant absorption effect with either containers, nor a measurable loss of potency due to degradation of the drug. However, solutions should be freshly prepared and used within 24 hours. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
