Drug Catalog - Product Detail
BUDESONIDE CAPS. 3MG CP 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0778-10 | AMNEAL PHARMACEUTICALS | 100 | 3MG | CAPSULE |
PACKAGE FILES
Generic Name
BUDESONIDE
Substance Name
BUDESONIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA206200
Description
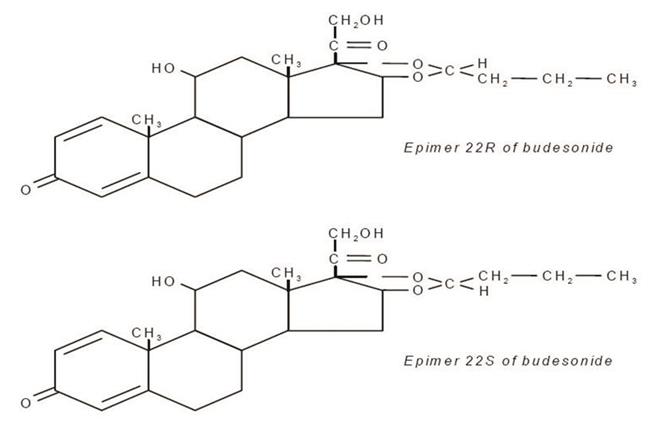
11 DESCRIPTION Budesonide USP, the active ingredient of budesonide delayed-release capsules, is a synthetic corticosteroid. Budesonide, USP is designated chemically as (RS)-11β, 16α, 17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide, USP is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide, USP is C 25 H 34 O 6 and its molecular weight is 430.5. Its structural formula is: Budesonide, USP is a white to off-white, tasteless, odorless powder that is practically insoluble in water and heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 5 is 1.6 x 10 3 ionic strength 0.01. Each delayed-release capsule for oral administration contains 3 mg of micronized budesonide, USP with the following inactive ingredients: cetyl alcohol, corn starch, ethyl cellulose, hypromellose, macrogol, methacrylic acid copolymer type C, sodium lauryl sulfate, sugar, talc and triethyl citrate. The capsule shells have the following inactive ingredients: D&C yellow #10, FD&C blue #1, FD&C red #40, FD&C yellow #6, gelatin and titanium dioxide. The monogramming ink has the following inactive ingredients: D&C yellow #10, ethanol, FD&C blue #1, FD&C blue #2, FD&C red #40, iron oxide black, methanol, N-butyl alcohol, propylene glycol and shellac. Structural Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Budesonide delayed-release capsules, 3 mg, are supplied as hard gelatin capsules with an opaque light gray body imprinted “778” and an opaque maroon cap imprinted “AMNEAL” with black ink. They are available as follows: Bottles of 21: NDC 65162-778-49 Bottles of 100: NDC 65162-778-10 Bottles of 180: NDC 65162-778-18 Bottles of 300: NDC 65162-778-30 Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed.
Indications & Usage
1 INDICATIONS AND USAGE Budesonide delayed-release capsules are a corticosteroid indicated for: Treatment of mild to moderate active Crohn’s disease involving the ileum and/or the ascending colon, in patients 8 years and older. (1.1) Maintenance of clinical remission of mild to moderate Crohn’s disease involving the ileum and/or the ascending colon for up to 3 months in adults. (1.2) 1.1 Treatment of Mild to Moderate Active Crohn’s Disease Budesonide delayed-release capsules are indicated for the treatment of mild to moderate active Crohn's disease involving the ileum and/or the ascending colon in patients 8 years of age and older. 1.2 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease Budesonide delayed-release capsules are indicated for the maintenance of clinical remission of mild to moderate Crohn’s disease involving the ileum and/or the ascending colon for up to 3 months in adults.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Administration Instructions (2.1) : Take once daily in the morning. Swallow whole. Do not chew or crush. For patients unable to swallow an intact capsule, open the capsules and empty the granules onto one tablespoonful of applesauce. Mix and consume the entire contents within 30 minutes. Do not chew or crush. Follow with 8 ounces of water. Avoid consumption of grapefruit juice for the duration of therapy. Recommended Dosage: Mild to moderate active Crohn’s disease (2.2) : Adults: 9 mg once daily for up to 8 weeks; repeat 8 week treatment courses for recurring episodes of active disease. Pediatrics 8 to 17 years who weigh more than 25 kg: 9 mg once daily for up to 8 weeks, followed by 6 mg once daily in the morning for 2 weeks. Maintenance of clinical remission of mild to moderate Crohn’s disease (2.3) : Adults: 6 mg once daily for up to 3 months; taper to complete cessation after 3 months. Continued treatment for more than 3 months has not been shown to provide substantial clinical benefit. When switching from oral prednisolone, begin tapering prednisolone concomitantly with initiating budesonide delayed-release capsules. Hepatic Impairment: Consider reducing the dosage to 3 mg once daily in adult patients with moderate hepatic impairment (Child-Pugh Class B). (2.4, 5.1, 8.6) 2.1 Administration Instructions Take budesonide delayed-release capsules once daily in the morning. Swallow budesonide delayed-release capsules whole. Do not chew or crush. For patients unable to swallow an intact capsule, budesonide delayed-release capsules can be opened and administered as follows: Place one tablespoonful of applesauce into a clean container (e.g., empty bowl). The applesauce used should not be hot and should be soft enough to be swallowed without chewing. Open the capsule(s). Carefully empty all the granules inside the capsule(s) on the applesauce. Mix the granules with the applesauce. Consume the entire contents within 30 minutes of mixing. Do not chew or crush the granules. Do not save the applesauce and granules for future use. Follow the applesauce and granules immediately with a glass (8 ounces) of cool water to ensure complete swallowing of the granules. Avoid consumption of grapefruit juice for the duration of budesonide delayed-release capsules therapy [see Drug Interactions (7.1) ] . 2.2 Treatment of Mild to Moderate Active Crohn's Disease The recommended dosage of budesonide delayed-release capsules are: Adults: 9 mg orally once daily for up to 8 weeks. Repeated 8 week courses of budesonide delayed-release capsules can be given for recurring episodes of active disease. Pediatric patients 8 to 17 years who weigh more than 25 kg: 9 mg orally once daily for up to 8 weeks, followed by 6 mg once daily for 2 weeks. 2.3 Maintenance of Clinical Remission of Mild to Moderate Crohn’s Disease The recommended dosage in adults, following an 8 week course(s) of treatment for active disease and once the patient’s symptoms are controlled (CDAI less than 150), is budesonide delayed-release capsules 6 mg orally once daily for maintenance of clinical remission up to 3 months. If symptom control is still maintained at 3 months an attempt to taper to complete cessation is recommended. Continued treatment with budesonide delayed-release capsules 6 mg for more than 3 months has not been shown to provide substantial clinical benefit. Patients with mild to moderate active Crohn’s disease involving the ileum and/or ascending colon have been switched from oral prednisolone to budesonide delayed-release capsules with no reported episodes of adrenal insufficiency. Since prednisolone should not be stopped abruptly, tapering should begin concomitantly with initiating budesonide delayed-release capsules treatment. 2.4 Dosage Adjustment in Adult Patients with Hepatic Impairment Consider reducing the dosage of budesonide delayed-release capsules to 3 mg once daily for adult patients with moderate hepatic impairment (Child-Pugh Class B). Avoid use in patients with severe hepatic impairment (Child-Pugh Class C) [see Warnings and Precautions (5.1), Use in Specific Populations (8.6) ] .
