Drug Catalog - Product Detail
BISOPROLOL FUMARATE TB 5MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 29300-0126-13 | UNICHEM PHARMACEUTICALS | 30 | 5MG | TABLET |
PACKAGE FILES

Generic Name
BISOPROLOL FUMARATE
Substance Name
BISOPROLOL FUMARATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078635
Description
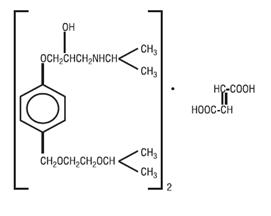
DESCRIPTION BISOPROLOL FUMARATE USP is a synthetic, beta 1 -selective (cardioselective) adrenoceptor blocking agent. The chemical name for bisoprolol fumarate is (±)-1-[4-[[2-(1-Methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-2-propanol (E)-2-butenedioate (2:1) (salt). It possesses an asymmetric carbon atom in its structure and is provided as a racemic mixture. The S(-) enantiomer is responsible for most of the beta-blocking activity. Its molecular formula is (C 18 H 31 NO 4 ) 2 •C 4 H 4 O 4 and its structure is: Bisoprolol fumarate has a molecular weight of 766.97. It is a white crystalline powder which is approximately equally hydrophilic and lipophilic, and is readily soluble in water, methanol, ethanol, and chloroform. BISOPROLOL FUMARATE TABLETS USP are available as 5 and 10 mg tablets for oral administration. Inactive ingredients include Colloidal Silicon Dioxide, Corn Starch (Pregelatinized), Crospovidone, Dibasic Calcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Polysorbate 80 and Titanium Dioxide. The 5 mg tablets also contain Red and Yellow Iron Oxide. Structure
How Supplied
HOW SUPPLIED BISOPROLOL FUMARATE is supplied as 5 mg and 10 mg tablets. The 5 mg tablet is pink colored, biconvex, round, film coated tablet debossed with UL on one side and scored on the other side with 5 debossed on either side of the score. Bottles of 30: NDC 29300-126-13 Bottles of 100: NDC 29300-126-01 Bottles of 500: NDC 29300-126-05 The 10 mg tablet is white colored, biconvex, round, film coated tablet debossed with UL on one side and 10 on the other side. Bottles of 30: NDC 29300-127-13 Bottles of 100: NDC 29300-127-01 Bottles of 500: NDC 29300-127-05 Store at 20° to 25°C (68° to 77°F). [see USP Controlled Room Temperature]. Protect from moisture. Dispense in tight, light-resistant containers. Please address medical inquiries to Unichem's toll free # 1-866-562-4616.
Indications & Usage
INDICATIONS AND USAGE BISOPROLOL FUMARATE is indicated in the management of hypertension. It may be used alone or in combination with other antihypertensive agents.
Dosage and Administration
DOSAGE AND ADMINISTRATION The dose of BISOPROLOL FUMARATE must be individualized to the needs of the patient. The usual starting dose is 5 mg once daily. In some patients, 2.5 mg may be an appropriate starting dose ( see Bronchospastic Disease in WARNINGS ). If the antihypertensive effect of 5 mg is inadequate, the dose may be increased to 10 mg and then, if necessary, to 20 mg once daily. Patients with Renal or Hepatic Impairment In patients with hepatic impairment (hepatitis or cirrhosis) or renal dysfunction (creatinine clearance less than 40 mL/min), the initial daily dose should be 2.5 mg and caution should be used in dose-titration. Since limited data suggest that bisoprolol fumarate is not dialyzable, drug replacement is not necessary in patients undergoing dialysis. Geriatric Patients It is not necessary to adjust the dose in the elderly, unless there is also significant renal or hepatic dysfunction (see above and Geriatric Use in PRECAUTIONS ) . Pediatric Patients There is no pediatric experience with BISOPROLOL FUMARATE.
