Drug Catalog - Product Detail
BENZONATATE USP DBL. STR. 200MG SG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69452-0144-20 | BIONPHARMA | 100 | 200MG | CAPSULE |
PACKAGE FILES




Generic Name
BENZONATATE
Substance Name
BENZONATATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA081297
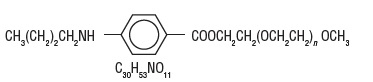
Description
DESCRIPTION Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p (butylamino) benzoate; with a molecular weight of 603.7. Each soft gelatin capsule, for oral administration, contains 100 mg or 200 mg benzonatate USP. In addition, each capsule contains the following inactive ingredients: D&C Yellow No. 10, gelatin, glycerin, and purified water. Structural Formula
How Supplied
HOW SUPPLIED Benzonatate Capsules USP, 100 mg: Yellow soft gelatin capsules, imprinted with "PA46", available in bottles of 100's (NDC 69452-143-20) and 500's (NDC 69452-143-30). Benzonatate Capsules USP, 200 mg: Yellow soft gelatin capsules, imprinted with "PA83", available in bottles of 100's (NDC 69452-144-20) and 500's (NDC 69452-144-30). The capsules should be protected from light, moisture and humidity, and stored at controlled room temperature 20° to 25°C (68° to 77°F) [See USP]. Dispense in a tight, light-resistant container as defined in USP/NF. Manufactured for: Bionpharma Inc. Princeton, NJ 08540, USA Rev. 12/2022 For India Manufacturing Site: Manufactured for: Bionpharma Inc. Princeton, NJ 085 40, USA MADE IN INDIA Rev. 08/2024 Code: TN/DRUGS/TN/25/00074 P3173/00/24
Indications & Usage
INDICATIONS AND USAGE BENZONATATE is indicated for the symptomatic relief of cough.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adults and Children over 10 years of age: Usual dose is one 100 mg or 200 mg capsule three times a day as needed for cough. If necessary to control cough, up to 600 mg daily in three divided doses may be given. BENZONATATE should be swallowed whole. BENZONATATE Capsules are not to be broken, chewed, dissolved, cut or crushed.
