Drug Catalog - Product Detail
BENAZEPRIL HCL TB 40MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43547-0338-50 | SOLCO HEALTHCARE | 500 | 40MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
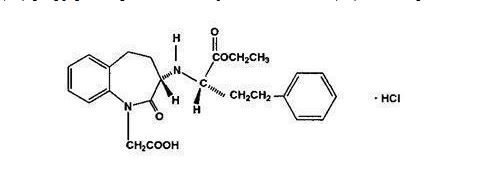
DESCRIPTION Benazepril hydrochloride is a white to off-white crystalline powder, soluble (>100 mg/mL) in water, in ethanol, and in methanol. Its chemical name is benazepril 3-[[1-(ethoxy-carbonyl)-3-phenyl-(1S)-propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid monohydrochloride; its structural formula is Its empirical formula is C 24 H 28 N 2 O 5 •HCl and its molecular weight is 460.96. Benazeprilat, the active metabolite of benazepril, is a non-sulfhydryl angiotensin-converting enzyme inhibitor. Benazepril is converted to benazeprilat by hepatic cleavage of the ester group. Benazepril hydrochloride, USP is supplied as film-coated tablets containing 5 mg, 10 mg, 20 mg, and 40 mg of benazepril hydrochloride for oral administration. The inactive ingredients are carnauba wax, colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, pregelatinized starch, titanium dioxide, and triacetin. The 10 mg tablet also contains FD&C Red No. 40 aluminum lake. The 20 mg tablet also contains black iron oxide and yellow iron oxide. The 40 mg tablet also contains FD&C Blue No. 2 aluminum lake. Benazepril hydrochloride tablets USP, 5 mg, 10 mg, 20 mg and 40 mg meet USP Dissolution Test 2. structure
How Supplied
HOW SUPPLIED Benazepril hydrochloride tablets, USP, 5 mg, are round, white, film-coated tablets, debossed “S” on one side and “341” on the other side, packaged as follows: NDC 43547-335-10 bottle of 100 tablets (with desiccant) Benazepril hydrochloride tablets, USP, 10 mg, are round, red, film-coated tablets, debossed “S” on one side and “342” on the other side, packaged as follows: NDC 43547-336-10 bottle of 100 tablets (with desiccant) NDC 43547-336-50 bottle of 500 tablets (with desiccant) Benazepril hydrochloride tablets, USP, 20 mg, are round, grey, film-coated tablets, debossed “S” on one side and “343” on the other side, packaged as follows: NDC 43547-337-10 bottle of 100 tablets (with desiccant) NDC 43547-337-50 bottle of 500 tablets (with desiccant) Benazepril hydrochloride tablets, USP, 40 mg, are round, blue, film-coated tablets, debossed “S” on one side and “344” on the other side, packaged as follows: NDC 43547-338-10 bottle of 100 tablets (with desiccant) NDC 43547-338-50 bottle of 500 tablets (with desiccant) Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Protect from moisture. Dispense in tight container (USP). To report SUSPECTED ADVERSE REACTIONS, contact Solco Healthcare US, LLC at 1-866-257-2597 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch Distributed by: Solco Healthcare US, LLC Cranbury, NJ 08512, USA Manufactured by: Zhejiang Huahai Pharmaceutical Co., Ltd. Xunqiao, Linhai, Zhejiang 317024, China Version: 05/2015 07713-02
Indications & Usage
INDICATIONS AND USAGE Benazepril hydrochloride tablets, USP are indicated for the treatment of hypertension. It may be used alone or in combination with thiazide diuretics.
Dosage and Administration
DOSAGE AND ADMINISTRATION Hypertension Adults The recommended initial dose for patients not receiving a diuretic is 10 mg once a day. The usual maintenance dosage range is 20-40 mg per day administered as a single dose or in two equally divided doses. A dose of 80 mg gives an increased response, but experience with this dose is limited. The divided regimen was more effective in controlling trough (pre-dosing) blood pressure than the same dose given as a once-daily regimen. Dosage adjustment should be based on measurement of peak (2-6 hours after dosing) and trough responses. If a once-daily regimen does not give adequate trough response, an increase in dosage or divided administration should be considered. If blood pressure is not controlled with benazepril hydrochloride tablets alone, a diuretic can be added. Total daily doses above 80 mg have not been evaluated. Concomitant administration of benazepril hydrochloride tablets with potassium supplements, potassium salt substitutes, or potassium-sparing diuretics can lead to increases of serum potassium (see PRECAUTIONS ). In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally can occur following the initial dose of benazepril hydrochloride tablets. To reduce the likelihood of hypotension, the diuretic should, if possible, be discontinued two to three days prior to beginning therapy with benazepril hydrochloride tablets (see WARNINGS ). Then, if blood pressure is not controlled with benazepril hydrochloride tablets alone, diuretic therapy should be resumed. If the diuretic cannot be discontinued, an initial dose of 5 mg benazepril should be used to avoid excessive hypotension. Pediatrics In children, doses of benazepril hydrochloride between 0.1 and 0.6 mg/kg once daily have been studied, and doses greater than 0.1 mg/kg were shown to reduce blood pressure (see Pharmacodynamics ). Based on this, the recommended starting dose of benazepril hydrochloride is 0.2 mg/kg once per day as monotherapy. Doses above 0.6 mg/kg (or in excess of 40 mg daily) have not been studied in pediatric patients. For pediatric patients who cannot swallow tablets, or for whom the calculated dosage (mg/kg) does not correspond to the available tablet strengths for benazepril hydrochloride tablets, follow the suspension preparation instructions below to administer benazepril HCL as a suspension. Treatment with benazepril hydrochloride tablets is not advised for children below the age of 6 years (see PRECAUTIONS , Pediatric Use ) and in pediatric patients with glomerular filtration rate <30 mL, as there are insufficient data available to support a dosing recommendation in these groups. For Hypertensive Patients with Renal Impairment For patients with a creatinine clearance <30 mL/min/1.73 m 2 (serum creatinine >3 mg/dL), the recommended initial dose is 5 mg benazepril hydrochloride tablet once daily. Dosage may be titrated upward until blood pressure is controlled or to a maximum total daily dose of 40 mg (see WARNINGS ). Preparation of Suspension (for 150 mL of a 2 mg/mL suspension) Add 75 mL of Ora-Plus ®* oral suspending vehicle to an amber polyethylene terephthalate (PET) bottle containing fifteen benazepril hydrochloride 20 mg tablets, and shake for at least 2 minutes. Allow the suspension to stand for a minimum of 1 hour. After the standing time, shake the suspension for a minimum of 1 additional minute. Add 75 mL of Ora-Sweet ®* oral syrup vehicle to the bottle and shake the suspension to disperse the ingredients. The suspension should be refrigerated at 2-8°C (36-46°F) and can be stored for up to 30 days in the PET bottle with a child-resistant screw-cap closure. Shake the suspension before each use. * Ora-Plus ® and Ora-Sweet ® are registered trademarks of Paddock Laboratories, Inc. Ora-Plus ® contains carrageenan, citric acid, methylparaben, microcrystalline cellulose, carboxymethylcellulose sodium, potassium sorbate, simethicone, sodium phosphate monobasic, xanthan gum, and water. Ora-Sweet ® contains citric acid, berry citrus flavorant, glycerin, methylparaben, potassium sorbate, sodium phosphate monobasic, sorbitol, sucrose, and water.
