Drug Catalog - Product Detail
AZITHROMYCIN ORAL SUSP. 200MG/5ML 1200MG
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 42806-0151-34 | EPIC PHARMA | 30 | 200MG/5ML | SUSPENSION |
PACKAGE FILES






Generic Name
AZITHROMYCIN
Substance Name
AZITHROMYCIN MONOHYDRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207531
Description
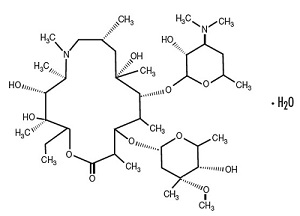
11 DESCRIPTION Azithromycin for oral suspension USP contains the active ingredient azithromycin monohydrate, USP, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one monohydrate. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C 38 H 72 N 2 O 12 ∙H2O, and its molecular weight is 767.00. Azithromycin has the following structural formula: Azithromycin, USP, as the monohydrate, is a white to off-white crystalline powder with a molecular formula of C 38 H 72 N 2 O 12 •H2O and a molecular weight of 767.00. Azithromycin for Oral Suspension USP is supplied in bottles containing azithromycin monohydrate powder equivalent to 300 mg, 600 mg, 900 mg, or 1200 mg azithromycin, USP per bottle and the following inactive ingredients: colloidal silicon dioxide, FD & C Red No. 40 Aluminum Lake, hydroxypropyl cellulose, sodium phosphate tribasic anhydrous, sucrose, natural and artificial banana flavor, natural and artificial cherry flavor and xanthan gum. After constitution, each 5 mL of suspension contains 100 mg or 200 mg of azithromycin, USP. The dry powder before constitution is off-white to pinkish in color. The suspension after constitution is pink to red in color. structure-formula.jpg
How Supplied
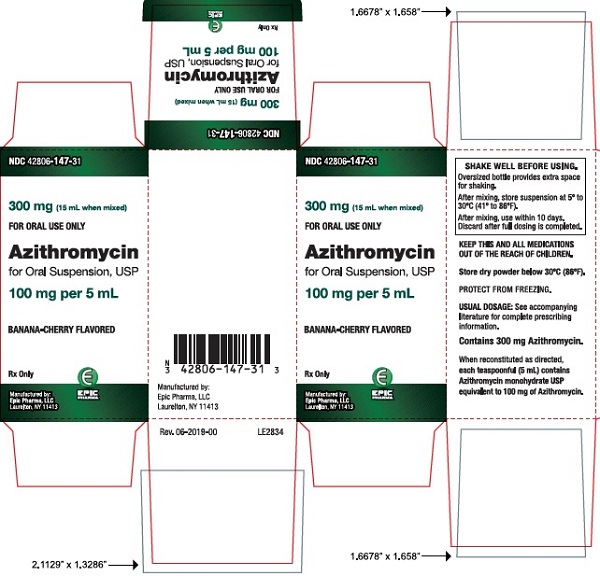
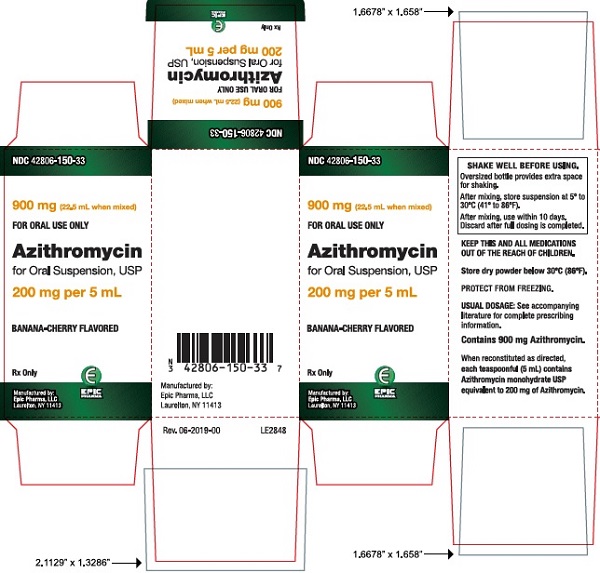
16 HOW SUPPLIED/STORAGE AND HANDLING Azithromycin for oral suspension USP after constitution contains a banana-cherry flavored suspension. Azithromycin for oral suspension USP is supplied to provide 100 mg/5 mL or 200 mg/5 mL suspension in bottles as follows: Azithromycin contents per bottle NDC 300 mg (15 mL bottle) 42806-147-31 600 mg (15 mL bottle) 42806-149-32 900 mg (22.5 mL bottle) 42806-150-33 1200 mg (30 mL bottle) 42806-151-34 [see Dosage and Administration (2) ] for constitution instructions with each bottle type. Storage: Store dry powder below 30°C (86°F). Store constituted suspension between 5° to 30°C (41° to 86°F) and discard when full dosing is completed.
Indications & Usage
1 INDICATIONS AND USAGE Azithromycin for oral suspension USP is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. Recommended dosages and durations of therapy in adult and pediatric patient populations vary in these indications. [see Dosage and Administration (2) ] Azithromycin for oral suspension USP is a macrolide antibacterial drug indicated for mild to moderate infections caused by designated, susceptible bacteria: • Acute bacterial exacerbations of chronic bronchitis in adults ( 1.1 ) • Acute bacterial sinusitis in adults ( 1.1 ) • Uncomplicated skin and skin structure infections in adults ( 1.1 ) • Urethritis and cervicitis in adults ( 1.1 ) • Genital ulcer disease in men ( 1.1 ) • Acute otitis media in pediatric patients (6 months of age and older) ( 1.2 ) • Community-acquired pneumonia in adults and pediatric patients (6 months of age and older) ( 1.1 , 1.2 ) • Pharyngitis/tonsillitis in adults and pediatric patients (2 years of age and older) ( 1.1 , 1.2 ) Limitation of Use : Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors. ( 1.3 ) To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. ( 1.4 ) 1.1 Adult Patients • Acute bacterial exacerbations of chronic bronchitis due to Hae mophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae . • Acute bacterial sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae . • Community-acquired pneumonia due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, or Streptococcus pneumoniae in patients appropriate for oral therapy. • Pharyngitis/tonsillitis caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy. • Uncomplicated skin and skin structure infections due to Staphylococcus aureus, Streptococcus pyogenes, or Streptococcus agalactiae . • Urethritis and cervicitis due to Chlamydia trachomatis or Neisseria gonorrhoeae . • Genital ulcer disease in men due to Haemophilus ducreyi (chancroid). Due to the small number of women included in clinical trials, the efficacy of azithromycin in the treatment of chancroid in women has not been established. 1.2 Pediatric Patients [see Use in Specific Populations (8.4) and Clinical Studies (14.2) ] • Acute otitis media (> 6 months of age ) caused by Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae . • Community-acquired pneumonia ( >6 months of age ) due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, or Streptococcus pneumoniae in patients appropriate for oral therapy. • Pharyngitis/tonsillitis ( >2 years of age ) caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy. 1.3 Limitations of Use Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following: • patients with cystic fibrosis, • patients with nosocomial infections, • patients with known or suspected bacteremia, • patients requiring hospitalization, • elderly or debilitated patients, or • patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia). 1.4 Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Adult Patients ( 2.1 ) Infection Recommended Dose/Duration of Therapy Community-acquired pneumonia (mild severity) Pharyngitis/tonsillitis (second-line therapy) Skin/skin structure (uncomplicated) 500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5. Acute bacterial exacerbations of chronic bronchitis (mild to moderate) 500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 or 500 mg once daily for 3 days. Acute bacterial sinusitis 500 mg once daily for 3 days. Genital ulcer disease (chancroid) Non-gonococcal urethritis and cervicitis One single 1 gram dose. Gonococcal urethritis and cervicitis One single 2 gram dose. • Pediatric Patients ( 2.2 ) Infection Recommended Dose/Duration of Therapy Acute otitis media (6 months of age and older) 30 mg/kg as a single dose or 10 mg/kg once daily for 3 days or 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg/day on Days 2 through 5. Acute bacterial sinusitis (6 months of age and older) 10 mg/kg once daily for 3 days. Community-acquired pneumonia (6 months of age and older) 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg once daily on Days 2 through 5. Pharyngitis/tonsillitis (2 years of age and older) 12 mg/kg once daily for 5 days. 2.1 Adult Patients [see Indications and Usage (1.1) and Clinical Pharmacology (12.3) ] Infection * Recommended Dose/Duration of Therapy Community-acquired pneumonia Pharyngitis/tonsillitis (second-line therapy) Skin/skin structure (uncomplicated) 500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 Acute bacterial exacerbations of chronic obstructive pulmonary disease 500 mg once daily for 3 days OR 500 mg as a single dose on Day 1, followed by 250 mg once daily on Days 2 through 5 Acute bacterial sinusitis 500 mg-once daily for 3 days Genital ulcer disease (chancroid) One single 1 gram dose Non-gonococcal urethritis and cervicitis One single 1 gram dose Gonococcal urethritis and cervicitis One single 2 gram dose * DUE TO THE INDICATED ORGANISMS [see Indications and Usage (1.1) ] Azithromycin tablets can be taken with or without food. 2.2 Pediatric Patients 1 Infection * Recommended Dose/Duration of Therapy Acute otitis media 30 mg/kg as a single dose or 10 mg/kg once daily for 3 days or 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg/day on Days 2 through 5. Acute bacterial sinusitis 10 mg/kg once daily for 3 days. Community-acquired pneumonia 10 mg/kg as a single dose on Day 1 followed by 5 mg/kg once daily on Days 2 through 5. Pharyngitis/tonsillitis 12 mg/kg once daily for 5 days. *DUE TO THE INDICATED ORGANISMS [see Indications and Usage (1.2) ] 1 see dosing tables below for maximum doses evaluated by indication Azithromycin for oral suspension can be taken with or without food. PEDIATRIC DOSAGE GUIDELINES FOR OTITIS MEDIA, ACUTE BACTERIAL SINUSITIS, AND COMMUNITY-ACQUIRED PNEUMONIA (Age 6 months and above, [see Use in Specific Populations (8.4) ]) Based on Body Weight OTITIS MEDIA AND COMMUNITY-ACQUIRED PNEUMONIA: (5-Day Regimen) * Dosing Calculated on 10 mg/kg/day Day 1 and 5 mg/kg/day Days 2 to 5. Weight 100 mg/5 mL 200 mg/5 mL Total mL per Treatment Course Total mg per Treatment Course Kg Day 1 Days 2-5 Day 1 Days 2-5 5 2.5 mL; (½ tsp) 1.25 mL; (¼ tsp) 7.5 mL 150 mg 10 5 mL; (1 tsp) 2.5 mL; (½ tsp) 15 mL 300 mg 20 5 mL; (1 tsp) 2.5 mL; (½ tsp) 15 mL 600 mg 30 7.5 mL; (1½ tsp) 3.75 mL; (¾ tsp) 22.5 mL 900 mg 40 10 mL; (2 tsp) 5 mL; (1 tsp) 30 mL 1200 mg 50 and above 12.5 mL; (2½ tsp) 6.25 mL; (1¼ tsp) 37.5 mL 1500 mg * Effectiveness of the 3-day or 1-day regimen in pediatric patients with community-acquired pneumonia has not been established. OTITIS MEDIA AND ACUTE BACTERIAL SINUSITIS: (3-Day Regimen) * Dosing Calculated on 10 mg/kg/day. Weight 100 mg/5 mL 200 mg/5 mL Total mL per Treatment Course Total mg per Treatment Course Kg Days 1-3 Days 1-3 5 2.5 mL; (½ tsp) 7.5 mL 150 mg 10 5 mL; (1 tsp) 15 mL 300 mg 20 5 mL (1 tsp) 15 mL 600 mg 30 7.5 mL (1½ tsp) 22.5 mL 900 mg 40 10 mL (2 tsp) 30 mL 1200 mg 50 and above 12.5 mL (2½ tsp) 37.5 mL 1500 mg * Effectiveness of the 5-day or 1-day regimen in pediatric patients with acute bacterial sinusitis has not been established. OTITIS MEDIA: (1-Day Regimen) Dosing Calculated on 30 mg/kg as a single dose. Weight 200 mg/5 mL Total mL per Treatment Course Total mg per Treatment Course Kg 1-Day Regimen 5 3.75 mL; (¾ tsp) 3.75 mL 150 mg 10 7.5 mL; (1½ tsp) 7.5 mL 300 mg 20 15 mL; (3 tsp) 15 mL 600 mg 30 22.5 mL; (4½ tsp) 22.5 mL 900 mg 40 30 mL; (6 tsp) 30 mL 1200 mg 50 and above 37.5 mL; (7½ tsp) 37.5 mL 1500 mg The safety of re-dosing azithromycin in pediatric patients who vomit after receiving 30 mg/kg as a single dose has not been established. In clinical studies involving 487 patients with acute otitis media given a single 30 mg/kg dose of azithromycin, 8 patients who vomited within 30 minutes of dosing were re-dosed at the same total dose. Pharyngitis/Tonsillitis : The recommended dose of azithromycin for children with pharyngitis/tonsillitis is 12 mg/kg once daily for 5 days. (See chart below.) PEDIATRIC DOSAGE GUIDELINES FOR PHARYNGITIS/TONSILLITIS (Age 2 years and above, [see Use in Specific Populations (8.4) ]) Based on Body Weight PHARYNGITIS/TONSILLITIS: (5-Day Regimen) Dosing Calculated on 12 mg/kg/day for 5 days. Weight 200 mg/5 mL Total mL per Treatment Course Total mg per Treatment Course Kg Day 1-5 8 2.5 mL; (½ tsp) 12.5 mL 500 mg 17 5 mL; (1 tsp) 25 mL 1000 mg 25 7.5 mL; (1½ tsp) 37.5 mL 1500 mg 33 10 mL; (2 tsp) 50 mL 2000 mg 40 12.5 mL; (2½ tsp) 62.5 mL 2500 mg Constituting instructions for azithromycin Oral Suspension 300, 600, 900, 1200 mg bottles. The table below indicates the volume of water to be used for constitution: Amount of water to be added Total volume after constitution (azithromycin content) Azithromycin concentration after constitution 9 mL (300 mg) 15 mL (300 mg) 100 mg/5 mL 9 mL (600 mg) 15 mL (600 mg) 200 mg/5 mL 12 mL (900 mg) 22.5 mL (900 mg) 200 mg/5 mL 15 mL (1200 mg) 30 mL (1200 mg) 200 mg/5 mL Shake well before each use. Oversized bottle provides shake space. Keep tightly closed. After mixing, store suspension at 5° to 30°C (41° to 86°F) and use within 10 days. Discard after full dosing is completed.
