Drug Catalog - Product Detail
AZELASTINE HCL OPHTH. SOLUTION SOL 0.0005 6ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61314-0308-02 | SANDOZ | 6 | 0.05% | NA |
PACKAGE FILES
Generic Name
AZELASTINE HYDROCHLORIDE
Substance Name
AZELASTINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAOCULAR
Application Number
ANDA202305
Description
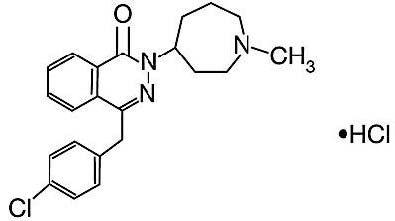
DESCRIPTION Azelastine Hydrochloride Ophthalmic Solution, 0.05% is a sterile ophthalmic solution containing azelastine hydrochloride, a relatively selective H 1 -receptor antagonist for topical administration to the eyes. Azelastine hydrochloride is a white crystalline powder with a molecular weight of 418.37. Azelastine hydrochloride is sparingly soluble in water, methanol and propylene glycol, and slightly soluble in ethanol, octanol, and glycerine. Azelastine hydrochloride is a racemic mixture with a melting point of 225°C. The chemical name for azelastine hydrochloride is (±)-1-(2H)-phthalazinone,4-[(4-chlorophenyl)methyl]-2- (hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride and is represented by the following chemical structure: Empirical chemical structure: C 22 H 24 ClN 3 O•HCl Each mL of Azelastine Hydrochloride Ophthalmic Solution, 0.05% contains: Active : 0.5 mg azelastine hydrochloride, equivalent to 0.457 mg of azelastine base; Preservative : 0.125 mg benzalkonium chloride; Inactives : disodium edetate dihydrate, hypromellose, sorbitol solution, sodium hydroxide and water for injection. It has a pH of approximately 5.0 to 6.5 and an osmolarity of approximately 271 to 312 mOsmol/L. Structure
How Supplied
HOW SUPPLIED Azelastine Hydrochloride Ophthalmic Solution, 0.05% is supplied sterile in a natural LDPE plastic bottle with a natural LDPE dropper tip and a white polypropylene cap as follows: 6 mL in 8 mL bottle NDC 61314-308-02 Storage Azelastine Hydrochloride Ophthalmic Solution, 0.05% 6 mL container: STORE UPRIGHT between 2° and 25°C (36° and 77°F). Rx Only Manufactured by Alcon Laboratories, Inc. Fort Worth, Texas 76134 for Sandoz Inc. Princeton, NJ 08540 Printed in USA Rev. August 2021 300049853-0821
Indications & Usage
INDICATIONS AND USAGE Azelastine Hydrochloride Ophthalmic Solution, 0.05% is indicated for the treatment of itching of the eye associated with allergic conjunctivitis.
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended dose is one drop instilled into each affected eye twice a day.
