Drug Catalog - Product Detail
ATOVAQUONE ORAL SUSPENSION SUSP 750MG/5ML 210ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0693-88 | AMNEAL PHARMACEUTICALS | 210 | 750MG/5ML | SUSPENSION |
PACKAGE FILES

Generic Name
ATOVAQUONE
Substance Name
ATOVAQUONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202960
Description
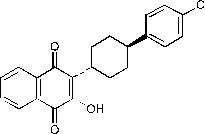
11 DESCRIPTION Atovaquone is a quinone antimicrobial drug for oral administration. The chemical name of atovaquone is trans -2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone, USP is a yellow crystalline solid that is practically insoluble in water. It has a molecular weight of 366.84 and the molecular formula C 22 H 19 ClO 3 . The compound has the following structural formula: Atovaquone oral suspension, USP is a formulation of micro-fine particles of atovaquone, USP. The atovaquone particles, reduced in size to facilitate absorption, are significantly smaller than those in the previously marketed tablet formulation. Atovaquone oral suspension, USP is for oral administration and is bright yellow with a citrus flavor. Each teaspoonful (5 mL) contains 750 mg of atovaquone, USP and the inactive ingredients benzyl alcohol, flavor (ethanol, propylene glycol, triacetin), poloxamer 188, purified water, saccharin sodium and xanthan gum. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Atovaquone oral suspension, USP (bright yellow, citrus-flavored) containing 750 mg atovaquone, USP in each teaspoonful (5 mL). Bottle of 210 mL with child-resistant cap (NDC 65162-693-88). Store at 15° to 25°C (59° to 77°F). Do not freeze . Dispense in tight container as defined in USP.
Indications & Usage
1 INDICATIONS AND USAGE Atovaquone oral suspension is a quinone antimicrobial drug indicated for: Prevention of Pneumocystis jirovecii pneumonia (PCP) in adults and adolescents aged 13 years and older who cannot tolerate trimethoprim-sulfamethoxazole (TMP-SMX). (1.1) Treatment of mild-to-moderate PCP in adults and adolescents aged 13 years and older who cannot tolerate TMP-SMX. (1.2) Limitations of Use (1.3) : Treatment of severe PCP (alveolar arterial oxygen diffusion gradient [(A-a)DO 2 ] >45 mm Hg) with atovaquone oral suspension has not been studied. The efficacy of atovaquone oral suspension in subjects who are failing therapy with TMP-SMX has also not been studied. 1.1 Prevention of Pneumocystis jirovecii Pneumonia Atovaquone oral suspension is indicated for the prevention of Pneumocystis jirovecii pneumonia (PCP) in adults and adolescents (aged 13 years and older) who cannot tolerate trimethoprim-sulfamethoxazole (TMP-SMX). 1.2 Treatment of Mild-to-Moderate Pneumocystis jirovecii Pneumonia Atovaquone oral suspension is indicated for the acute oral treatment of mild-to-moderate PCP in adults and adolescents (aged 13 years and older) who cannot tolerate TMP-SMX. 1.3 Limitations of Use Clinical experience with atovaquone oral suspension for the treatment of PCP has been limited to subjects with mild-to-moderate PCP (alveolar-arterial oxygen diffusion gradient [(A-a)DO 2 ] ≤45 mm Hg). Treatment of more severe episodes of PCP with atovaquone oral suspension has not been studied. The efficacy of atovaquone in subjects who are failing therapy with TMP-SMX has also not been studied.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Prevention of PCP: 1,500 mg (10 mL) once daily with food. (2.1) Treatment of PCP: 750 mg (5 mL) twice daily with food for 21 days. (2.2) Supplied in Bottles: Shake bottle gently before use. (2.3) 2.1 Dosage for the Prevention of P. jirovecii Pneumonia The recommended oral dosage is 1,500 mg (10 mL) once daily administered with food. 2.2 Dosage for the Treatment of Mild-to-Moderate P. jirovecii Pneumonia The recommended oral dosage is 750 mg (5 mL) twice daily (total daily dose = 1,500 mg) administered with food for 21 days. 2.3 Important Administration Instructions Administer atovaquone oral suspension with food to avoid low plasma atovaquone concentrations that may limit response to therapy [see Warnings and Precautions (5.1) , Clinical Pharmacology (12.3) ]. Atovaquone Oral Suspension Bottle Shake bottle gently before administering the recommended dosage.
