Drug Catalog - Product Detail
ATORVASTATIN 10MG 1000 TAB
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0944-15 | CIPLA USA | 1000 | 10MG | TABLET |
PACKAGE FILES





Generic Name
ATORVASTATIN CALCIUM
Substance Name
ATORVASTATIN CALCIUM TRIHYDRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA205519
Description
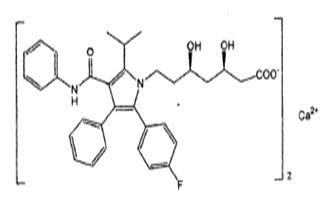
11 DESCRIPTION Atorvastatin calcium is a synthetic lipid-lowering agent. Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis. Atorvastatin calcium is [R-(R*,R*)]-2-(4-fluorophenyl)-ß,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1).The empirical formula of atorvastatin calcium is C 66 H 68 Ca F 2 N 4 O 10 and its molecular weight is 1155.36. Its structural formula is: Atorvastatin calcium is a white to off-white colored powder free from visible extraneous matter. Atorvastatin calcium is soluble in dimethyl sulphoxide, slightly soluble in alcohol, very slightly soluble in water, in pH 7.4 phosphate buffer and in acetonitrile and practically insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium tablets for oral administration contain 10, 20, 40, or 80 mg atorvastatin and the following inactive ingredients: anhydrous lactose, NF; colloidal silicon dioxide, NF; copovidone, NF; croscarmellose sodium, NF; magnesium stearate, NF; mannitol, USP; silicified microcrystalline cellulose, NF; sodium bicarbonate, USP; sodium carbonate anhydrous, NF; sodium lauryl sulfate, NF; lecitihin, polyvinyl alcohol part hydrolyzed, talc, titanium dioxide, xanthan gum and iron oxide yellow. Figure-01 Figure-02 Figure-03 chemical-structure
How Supplied
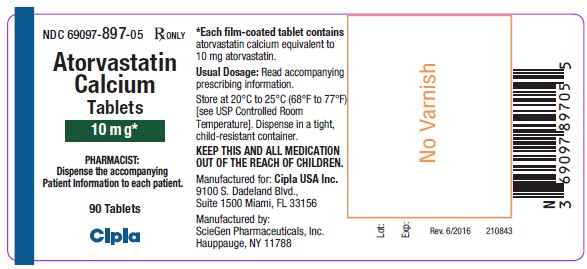
16 HOW SUPPLIED/STORAGE AND HANDLING 10 mg tablets: Yellow, oval shape, biconvex, film coated tablets, debossed with SG on one side and 152 on other side. Bottles of 90 NDC 69097-944-05 Bottles of 1000 NDC 69097-944-15 20 mg tablets: Yellow, oval shape, biconvex, film coated tablets, debossed with SG on one side and 153 on other side. Bottles of 90 NDC 69097-945-05 Bottles of 500 NDC 69097-945-12 40 mg Tablets: Yellow, oval shape, biconvex, film coated tablets, debossed with SG on one side and 154 on other side. Bottles of 90 NDC 69097-946-05 Bottles of 1000 NDC 69097-946-15 80 mg Tablets: Yellow, oval shape, biconvex, film coated tablets, debossed with SG on one side and 155 on other side. Bottles of 90 NDC 69097-947-05 Bottles of 500 NDC 69097-947-12 Storage Store at 20°C to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight, child-resistant container.
Indications & Usage
1 INDICATIONS AND USAGE Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is recommended as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In patients with CHD or multiple risk factors for CHD, atorvastatin calcium tablets can be started simultaneously with diet. Atorvastatin calcium tablet is an HMG-CoA reductase inhibitor indicated as an adjunct therapy to diet to: Reduce the risk of MI, stroke, revascularization procedures, and angina in adult patients without CHD, but with multiple risk factors ( 1.1 ). Reduce the risk of MI and stroke in adult patients with type 2 diabetes without CHD, but with multiple risk factors ( 1.1 ). Reduce the risk of non-fatal MI, fatal and non-fatal stroke, revascularization procedures, hospitalization for CHF, and angina in adult patients with CHD ( 1.1 ). Reduce elevated total-C, LDL-C, apo B, and TG levels and increase HDL-C in adult patients with primary hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia ( 1.2 ). Reduce elevated TG in adult patients with hypertriglyceridemia and primary dysbetalipoproteinemia ( 1.2 ). Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) ( 1.2 ). Reduce elevated total-C, LDL-C, and apo B levels in pediatric patients 10 years to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH) after failing an adequate trial of diet therapy ( 1.2 ). Limitations of Use: Atorvastatin calcium tablets has not been studied in Fredrickson Types I and V dyslipidemias ( 1.3 ). 1.1 Prevention of Cardiovascular Disease in Adults In adult patients without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as age, smoking, hypertension, low HDL-C, or a family history of early coronary heart disease, atorvastatin calcium tablets are indicated to: Reduce the risk of myocardial infarction Reduce the risk of stroke Reduce the risk for revascularization procedures and angina In adult patients with type 2 diabetes, and without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as retinopathy, albuminuria, smoking, or hypertension, atorvastatin calcium tablets are indicated to: Reduce the risk of myocardial infarction Reduce the risk of stroke In adult patients with clinically evident coronary heart disease, atorvastatin calcium tablets are indicated to: Reduce the risk of non-fatal myocardial infarction Reduce the risk of fatal and non-fatal stroke Reduce the risk for revascularization procedures Reduce the risk of hospitalization for CHF Reduce the risk of angina 1.2 Hyperlipidemia Atorvastatin calcium tablets are indicated: As an adjunct to diet to reduce elevated total-C, LDL-C, apo B, and TG levels and to increase HDL-C in adult patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia ( Fredrickson Types IIa and IIb); As an adjunct to diet for the treatment of adult patients with elevated serum TG levels ( Fredrickson Type IV); For the treatment of adult patients with primary dysbetalipoproteinemia ( Fredrickson Type III) who do not respond adequately to diet; To reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable; As an adjunct to diet to reduce total-C, LDL-C, and apo B levels in pediatric patients, 10 years to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH) if after an adequate trial of diet therapy the following findings are present: a. LDL-C remains ≥ 190 mg/dL or b. LDL-C remains ≥ 160 mg/dL and: there is a positive family history of premature cardiovascular disease or two or more other CVD risk factors are present in the pediatric patient 1.3 Limitations of Use Atorvastatin calcium tablets have not been studied in conditions where the major lipoprotein abnormality is elevation of chylomicrons ( Fredrickson Types I and V).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Dose range: 10 to 80 mg once daily ( 2.1 ). Recommended start dose: 10 or 20 mg once daily ( 2.1 ). Patients requiring large LDL-C reduction (>45%) may start at 40 mg once daily ( 2.1 ). Pediatric patients with HeFH: starting dose: 10 mg once daily; dose range: 10 to 20 mg/day for patients 10 years to 17 years of age ( 2.2 ). 2.1 Hyperlipidemia and Mixed Dyslipidemia The recommended starting dose of atorvastatin calcium tablets is 10 or 20 mg once daily. Patients who require a large reduction in LDL-C (more than 45%) may be started at 40 mg once daily. The dosage range of atorvastatin calcium tablets is 10 to 80 mg once daily. Atorvastatin calcium tablets can be administered as a single dose at any time of the day, with or without food. The starting dose and maintenance doses of atorvastatin calcium tablets should be individualized according to patient characteristics such as goal of therapy and response. After initiation and/or upon titration of atorvastatin calcium tablets, lipid levels should be analyzed within 2 to 4 weeks and dosage adjusted accordingly. 2.2 Heterozygous Familial Hypercholesterolemia in Pediatric Patients (10 Years to 17 Years of age) The recommended starting dose of atorvastatin calcium tablets is 10 mg/day; the usual dose range is 10 to 20 mg orally once daily [see Clinical Studies (14.6) ]. Doses should be individualized according to the recommended goal of therapy [see Indications and Usage (1.2) and Clinical Pharmacology (12) ] . Adjustments should be made at intervals of 4 weeks or more. 2.3 Homozygous Familial Hypercholesterolemia The dosage of atorvastatin calcium tablets in patients with homozygous HoFH is 10 to 80 mg daily. Atorvastatin calcium tablets should be used as an adjunct to other Lipid-lowering treatments (e.g., LDL apheresis) in these patients or if such treatments are unavailable. 2.4 Concomitant Lipid-Lowering Therapy Atorvastatin calcium tablets may be used with bile acid resins. The combination of HMG-CoA reductase inhibitors (statins) and fibrates should generally be used with caution [see Warnings and Precautions (5.1) and Drug Interactions (7) ] . 2.5 Dosage in Patients with Renal Impairment Renal disease does not affect the plasma concentrations nor LDL-C reduction of atorvastatin calcium tablets; thus, dosage adjustment in patients with renal dysfunction is not necessary [see Warnings and Precautions (5.1) , Clinical Pharmacology (12.3) ] . 2.6 Dosage in Patients Taking Cyclosporine, Clarithromycin, Itraconazole, or Certain Protease Inhibitors In patients taking cyclosporine or the HIV protease inhibitors (tipranavir plus ritonavir) or the hepatitis C protease inhibitor (telaprevir), therapy with atorvastatin calcium tablets should be avoided. In patients with HIV taking lopinavir plus ritonavir, caution should be used when prescribing atorvastatin calcium tablets and the lowest dose necessary employed. In patients taking clarithromycin, itraconazole, or in patients with HIV taking a combination of saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, or fosamprenavir plus ritonavir, therapy with atorvastatin calcium tablets should be limited to 20 mg, and appropriate clinical assessment is recommended to ensure that the lowest dose necessary of atorvastatin calcium is employed. In patients taking the HIV protease inhibitor nelfinavir or the hepatitis C protease inhibitor boceprevir, therapy with atorvastatin calcium tablets should be limited to 40 mg, and appropriate clinical assessment is recommended to ensure that the lowest dose necessary of atorvastatin calcium tablets is employed [see Warnings and Precautions (5.1) and Drug Interactions (7) ] .
