Drug Catalog - Product Detail
ATOMOXETINE HCL CAP 40MG 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 64980-0376-03 | RISING PHARMACEUTICALS | 30 | 40MG | CAPSULE |
PACKAGE FILES

Generic Name
ATOMOXETINE
Substance Name
ATOMOXETINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA079016
Description
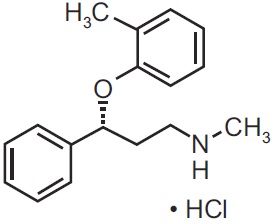
11 DESCRIPTION Atomoxetine is a selective norepinephrine reuptake inhibitor. Atomoxetine hydrochloride is the R (-) isomer as determined by x-ray diffraction. The chemical designation is (-)- N -Methyl-3-phenyl-3-( o -tolyloxy)-propylamine hydrochloride. The molecular formula is C 17 H 21 NO•HCl, which corresponds to a molecular weight of 291.82. The chemical structure is: Atomoxetine hydrochloride USP is a white to practically white solid, which has a solubility of 27.8 mg/mL in water. Atomoxetine capsules USP are intended for oral administration only. Each capsule contains atomoxetine hydrochloride USP equivalent to 10 mg, 18 mg, 25 mg, 40 mg, 60 mg, 80 mg, or 100 mg of atomoxetine. The capsules also contain the following inactive ingredients: pregelatinized starch and simethicone emulsion. The empty hard gelatin capsule shells contain gelatin, titanium dioxide, and sodium lauryl sulfate. In addition, the 18 mg contains iron oxide yellow, 25 mg and 40 mg contains FD&C Blue No 2, 60 mg contains FD&C Blue No 2 and iron oxide yellow, 80 mg and 100 mg contain iron oxide red and iron oxide yellow. The capsules are printed with edible ink containing black iron oxide and shellac. chemical structure
How Supplied
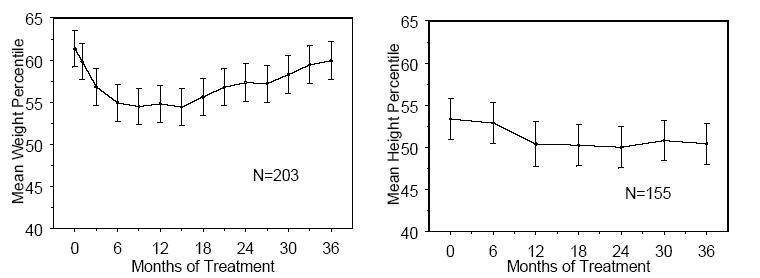
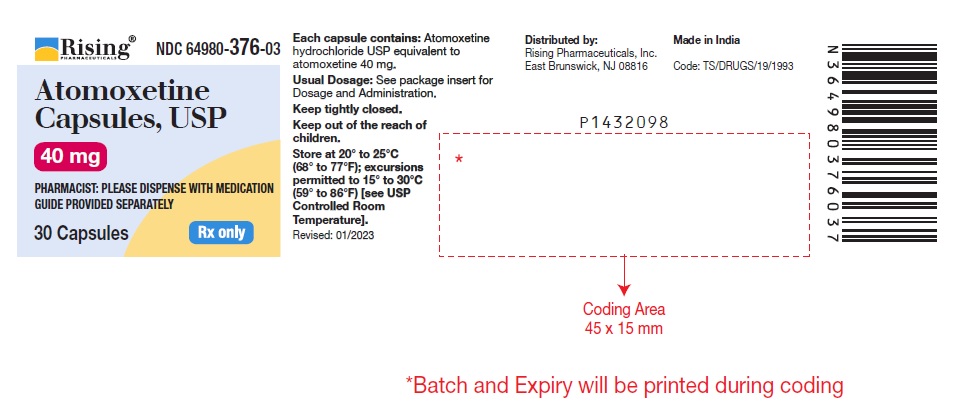
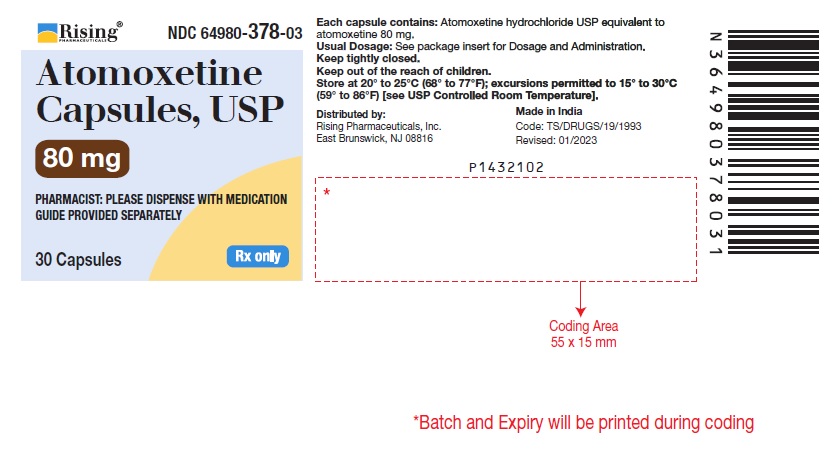
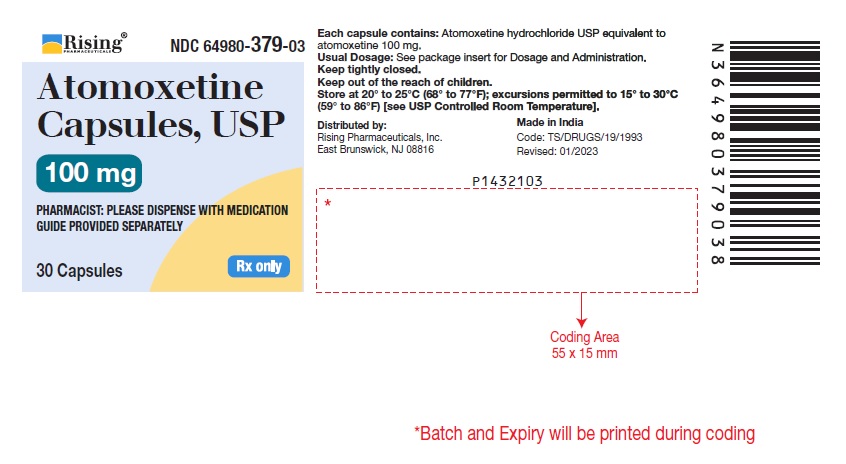
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Atomoxetine Capsules USP, 10 mg* are off-white opaque/off-white opaque, size ‘5’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘F’ on off-white opaque cap & ‘41’ on off-white opaque body with black ink. Bottles of 30 NDC 64980-373-03 Atomoxetine Capsules USP, 18 mg* are golden opaque/off-white opaque, size ‘4’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘F’ on golden opaque cap & ‘42’ on off-white opaque body with black ink. Bottles of 30 NDC 64980-374-03 Atomoxetine Capsules USP, 25 mg* are blue opaque/off-white opaque, size ‘4’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘F’ on blue opaque cap & ‘43’ on off-white opaque body with black ink. Bottles of 30 NDC 64980-375-03 Atomoxetine Capsules USP, 40 mg* are blue opaque/blue opaque, size ‘2’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘F’ on blue opaque cap & ‘45’ on blue opaque body with black ink. Bottles of 30 NDC 64980-376-03 Bottles of 1,000 NDC 64980-376-10 Atomoxetine Capsules USP, 60 mg* are blue opaque/golden opaque, size ‘1’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘F’ on blue opaque cap & ‘46’ on golden opaque body with black ink. Bottles of 30 NDC 64980-377-03 Bottles of 1,000 NDC 64980-377-10 Atomoxetine Capsules USP, 80 mg* are brown opaque/off-white opaque, size ‘0’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘F’ on brown opaque cap & ‘47’ on off-white opaque body with black ink. Bottles of 30 NDC 64980-378-03 Atomoxetine Capsules USP, 100 mg* are brown opaque/brown opaque, size ‘0EL’ hard gelatin capsule filled with white to off-white powder and imprinted with ‘Y’ on brown opaque cap & ‘04’ on brown opaque body with black ink. Bottles of 30 NDC 64980-379-03 * Atomoxetine base equivalent. 16.2 Storage and Handling Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Atomoxetine capsules are a selective norepinephrine reuptake inhibitor indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). (1.1) 1.1 Attention-Deficit/Hyperactivity Disorder (ADHD) Atomoxetine capsules are indicated for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD). The efficacy of atomoxetine capsules was established in seven clinical trials in outpatients with ADHD: four 6 to 9-week trials in pediatric patients (ages 6 to 18), two 10-week trial in adults, and one maintenance trial in pediatrics (ages 6 to 15) [see Clinical Studies (14) ] . 1.2 Diagnostic Considerations A diagnosis of ADHD (DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that cause impairment and that were present before age 7 years. The symptoms must be persistent, must be more severe than is typically observed in individuals at a comparable level of development, must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and must be present in 2 or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. The specific etiology of ADHD is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but also of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the patient and not solely on the presence of the required number of DSM-IV characteristics. For the Inattentive Type, at least 6 of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes, lack of sustained attention, poor listener, failure to follow through on tasks, poor organization, avoids tasks requiring sustained mental effort, loses things, easily distracted, forgetful. For the Hyperactive-Impulsive Type, at least 6 of the following symptoms must have persisted for at least 6 months: fidgeting/squirming, leaving seat, inappropriate running/climbing, difficulty with quiet activities, “on the go,” excessive talking, blurting answers, can’t wait turn, intrusive. For a Combined Type diagnosis, both inattentive and hyperactive-impulsive criteria must be met. 1.3 Need for Comprehensive Treatment Program Atomoxetine capsules are indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all patients with this syndrome. Drug treatment is not intended for use in the patient who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential in children and adolescents with this diagnosis and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe drug treatment medication will depend upon the physician’s assessment of the chronicity and severity of the patient’s symptoms.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Initial, Target and Maximum Daily Dose (2.1) (Acute and Maintenance/Extended Treatment) Body Weight Initial Daily Dose Target Total Daily Dose Maximum Total Daily Dose Children and adolescents up to 70 kg 0.5 mg/kg 1.2 mg/kg 1.4 mg/kg Children and adolescents over 70 kg and adults 40 mg 80 mg 100 mg Dosing adjustment — Hepatic Impairment, Strong CYP2D6 Inhibitor, and in patients known to be CYP2D6 poor metabolizers (PMs). ( 2.5 , 12.3) 2.1 Acute Treatment Dosing of children and adolescents up to 70 kg body weight — Atomoxetine capsules should be initiated at a total daily dose of approximately 0.5 mg/kg and increased after a minimum of 3 days to a target total daily dose of approximately 1.2 mg/kg administered either as a single daily dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. No additional benefit has been demonstrated for doses higher than 1.2 mg/kg/day [see Clinical Studies (14) ] . The total daily dose in children and adolescents should not exceed 1.4 mg/kg or 100 mg, whichever is less. Dosing of children and adolescents over 70 kg body weight and adults — Atomoxetine capsules should be initiated at a total daily dose of 40 mg and increased after a minimum of 3 days to a target total daily dose of approximately 80 mg administered either as a single daily dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. After 2 to 4 additional weeks, the dose may be increased to a maximum of 100 mg in patients who have not achieved an optimal response. There are no data that support increased effectiveness at higher doses [see Clinical Studies (14) ] . The maximum recommended total daily dose in children and adolescents over 70 kg and adults is 100 mg. 2.2 Maintenance/Extended Treatment It is generally agreed that pharmacological treatment of ADHD may be needed for extended periods. The benefit of maintaining pediatric patients (ages 6 to 15 years) with ADHD on atomoxetine capsules after achieving a response in a dose range of 1.2 to 1.8 mg/kg/day was demonstrated in a controlled trial. Patients assigned to atomoxetine capsules in the maintenance phase were generally continued on the same dose used to achieve a response in the open label phase. The physician who elects to use atomoxetine capsules for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient [see Clinical Studies (14.1) ] . 2.3 General Dosing Information Atomoxetine capsules may be taken with or without food. Atomoxetine capsules can be discontinued without being tapered. Atomoxetine capsules are not intended to be opened, they should be taken whole [see Patient Counseling Information (17) ] . The safety of single doses over 120 mg and total daily doses above 150 mg have not been systematically evaluated. 2.4 Screen for Bipolar Disorder Prior to Starting Atomoxetine Capsules Prior to initiating treatment with atomoxetine capsules, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.6) ]. 2.5 Dosing in Specific Populations Dosing adjustment for hepatically impaired patients — For those ADHD patients who have hepatic insufficiency (HI), dosage adjustment is recommended as follows: For patients with moderate HI (Child-Pugh Class B), initial and target doses should be reduced to 50% of the normal dose (for patients without HI). For patients with severe HI (Child-Pugh Class C), initial dose and target doses should be reduced to 25% of normal [see Use in Specific Populations (8.6) ] . Dosing adjustment for use with a strong CYP2D6 inhibitor or in patients who are known to be CYP2D6 PMs — In children and adolescents up to 70 kg body weight administered strong CYP2D6 inhibitors, e.g., paroxetine, fluoxetine, and quinidine, or in patients who are known to be CYP2D6 PMs, atomoxetine capsules should be initiated at 0.5 mg/kg/day and only increased to the usual target dose of 1.2 mg/kg/day if symptoms fail to improve after 4 weeks and the initial dose is well tolerated. In children and adolescents over 70 kg body weight and adults administered strong CYP2D6 inhibitors, e.g., paroxetine, fluoxetine, and quinidine, atomoxetine capsules should be initiated at 40 mg/day and only increased to the usual target dose of 80 mg/day if symptoms fail to improve after 4 weeks and the initial dose is well tolerated.
