Drug Catalog - Product Detail
ANAGRELIDE HCL CAP 1 MG 100 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 13668-0462-01 | TORRENT PHARMACEUTICALS | 100 | 1MG | CAPSULE |
PACKAGE FILES



Generic Name
ANAGRELIDE
Substance Name
ANAGRELIDE HYDROCHLORIDE ANHYDROUS
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA209151
Description
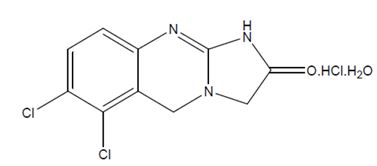
11 DESCRIPTION Anagrelide hydrochloride, USP is a platelet-reducing agent. Its chemical name is 6,7-dichloro-1,5 dihydroimidazo[2,1-b] quinazolin-2(3H)-one monohydrochloride monohydrate and it has the following structural formula: C 10 H 7 Cl 2 N 3 O·HCl·H 2 O M.W. 310.56 Anagrelide hydrochloride, USP is a white to off white powder that is practically insoluble in water and slightly soluble in dimethyl sulfoxide and very slightly soluble in dimethylformamide. Each Anagrelide Capsule USP, for oral administration, contains either 0.5 mg or 1 mg of anagrelide as anagrelide hydrochloride, USP and has the following inactive ingredients: black iron oxide, gelatin, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, hydroxypropyl cellulose, propylene glycol, shellac and titanium dioxide, potassium hydroxide. Image
How Supplied
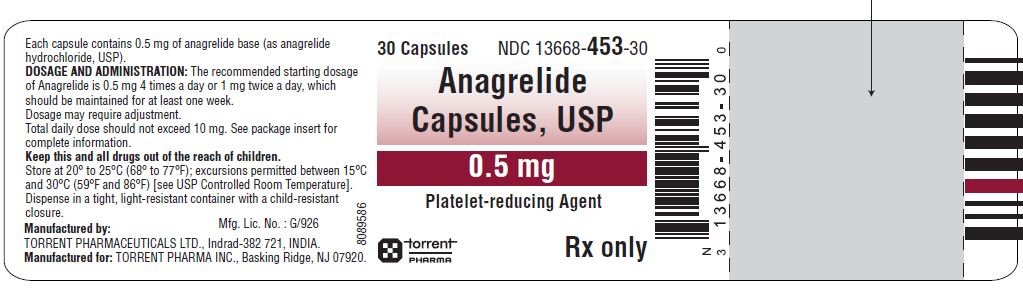
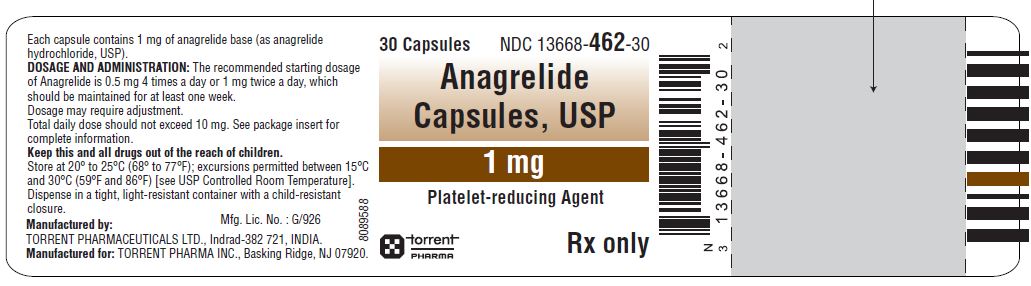
16 HOW SUPPLIED/STORAGE AND HANDLING Anagrelide capsules, USP 0.5 mg are Size '4', hard gelatin capsule having grey opaque cap and white opaque body, imprinted '1453' on cap and '0.5 mg' on body with black ink containing white to off-white powder. Bottles of 30 NDC 13668-453-30 Bottles of 100 NDC 13668-453-01 Anagrelide capsules, USP 1 mg are Size '3', hard gelatin capsule having White opaque cap and White opaque body, imprinted '1462' on cap and '1 mg' on body with black ink containing white to off-white powder. Bottles of 30 NDC 13668-462-30 Bottles of 100 NDC 13668-462-01 Store at 20 ° to 25 ° C (68 ° to 77 ° F); excursions permitted between 15 ° C and 30 ° C (59 ° F and 86 ° F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container with a child-resistant closure. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Indications & Usage
1 INDICATIONS AND USAGE Anagrelide is a platelet reducing agent indicated for the treatment of thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events. ( 1 ) Anagrelide capsules are indicated for the treatment of patients with thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events .
Dosage and Administration
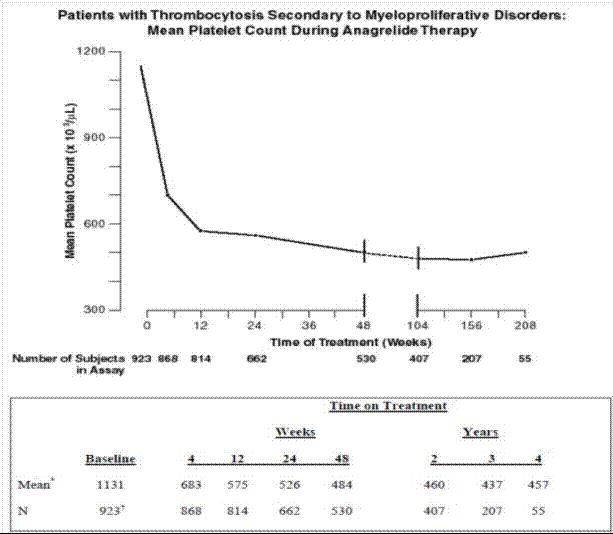
2 DOSAGE AND ADMINISTRATION The starting dose for adults is 0.5 mg four times a day or 1 mg twice a day. ( 2.1 ) The starting dose for pediatric patients is 0.5 mg per day. ( 2.1 ) Maintain the starting dose for at least one week and then titrate to maintain target platelet counts. ( 2.2 ) Do not exceed a dose increment of 0.5 mg/day in any one week. Do not exceed 10 mg/day or 2.5 mg in a single dose. ( 2.2 ) Moderate hepatic impairment: Start with 0.5 mg per day. ( 2.3 ) 2.1 Recommended Starting Dosage Adults: The recommended starting dosage of anagrelide capsules is 0.5 mg four times daily or 1 mg twice daily. Pediatric Patients: The recommended starting dosage of anagrelide capsules is 0.5 mg daily. 2.2 Dose Titration Based Upon Platelet Response Continue the starting dose for at least one week and then titrate to reduce and maintain the platelet count below 600,000/μL, and ideally between 150,000/μL and 400,000/μL. The dose increment should not exceed 0.5 mg/day in any one week. Dosage should not exceed 10 mg/day or 2.5 mg in a single dose. Most patients will experience an adequate response at a dose of 1.5 to 3.0 mg/day. Monitor platelet counts weekly during titration then monthly or as necessary. 2.3 Dose Modifications for Hepatic Impairment In patients with moderate hepatic impairment (Child Pugh score 7 to 9) start anagrelide capsules therapy at a dose of 0.5 mg/day and monitor frequently for cardiovascular events [see Warnings and Precautions (5.1), Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. Patients with moderate hepatic impairment who have tolerated anagrelide capsules therapy for one week may have their dose increased. The dose increase increment should not exceed 0.5 mg/day in any one week. Avoid use of anagrelide capsules in patients with severe hepatic impairment. 2.4 Clinical Monitoring Anagrelide capsules therapy requires clinical monitoring, including complete blood counts, assessment of hepatic and renal function, and electrolytes. To prevent the occurrence of thrombocytopenia, monitor platelet counts every two days during the first week of treatment and at least weekly thereafter until the maintenance dosage is reached. Typically, platelet counts begin to respond within 7 to 14 days at the proper dosage. In the clinical trials, the time to complete response, defined as platelet count ≤600,000/μL, ranged from 4 to 12 weeks. In the event of dosage interruption or treatment withdrawal, the rebound in platelet count is variable, but platelet counts typically will start to rise within 4 days and return to baseline levels in one to two weeks, possibly rebounding above baseline values. Monitor platelet counts frequently.
