Drug Catalog - Product Detail
AMPICILLIN ORAL SUSPENSION SUSP 250MG/5ML 100ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 67253-0183-10 | PAR PHARMACEUTICAL | 100 | 250MG/5ML | SUSPENSION |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
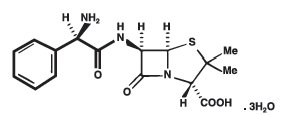
DESCRIPTION Ampicillin trihydrate is a semisynthetic penicillin derived from the basic penicillin nucleus, 6-aminopenicillanic acid. Ampicillin is designated chemically as (2S, 5R, 6R)-6-[(R)-2-Amino-2-phenylacetamido]-3, 3-dimethyl-7-oxo-4-thia-l-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate. It has the following chemical structure: The molecular formula is C 16 H 19 N 3 O 4 S.3H 2 O, and the molecular weight is 403.45. Ampicillin Capsules, USP for oral administration provide ampicillin trihydrate equivalent to 250 mg and 500 mg ampicillin. Ampicillin Capsules, USP also contains magnesium stearate, NF. The capsule shell contains black iron oxide; D&C red No. 28; FD&C blue No. 1; gelatin, NF; silicon dioxide, NF; sodium lauryl sulfate, NF; titanium dioxide USP. Ampicillin for Oral Suspension, USP provides ampicillin trihydrate equivalent to 125 mg/5 mL and 250 mg/5 mL ampicillin. Ampicillin for Oral Suspension, USP also contains flavors; microcrystalline cellulose and carboxymethylcellulose sodium, NF; colloidal silicon dioxide, NF; sodium citrate, USP; sodium propionate, NF; sucrose, NF Structure
How Supplied
HOW SUPPLIED Ampicillin Capsules, USP 250 mg : Each capsule contains ampicillin trihydrate equivalent to 250 mg ampicillin. The number 2 size capsule has a gray opaque body with a light blue opaque cap, printed WC402. Bottles of 100 NDC 67253-180-10 Bottles of 500 NDC 67253-180-50 Ampicillin Capsules, USP 500 mg : Each capsule contains ampicillin trihydrate equivalent to 500 mg ampicillin. The number 0 size capsule has a gray opaque body with a light blue opaque cap, printed WC404. Bottles of 100 NDC 67253-181-10 Bottles of 500 NDC 67253-181-50 Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Ampicillin for Oral Suspension, USP is available as a powder which when reconstituted as directed yields a white, bubble gum flavoured suspension. Ampicillin for Oral Suspension, USP 125 mg/5 mL: Each 5 mL of reconstituted suspension contains ampicillin trihydrate equivalent to 125 mg ampicillin. 100 mL bottles NDC 67253-182-10 200 mL bottles NDC 67253-182-20 Ampicillin for Oral Suspension, USP 250 mg/5 mL : Each 5 mL of reconstituted suspension contains ampicillin trihydrate equivalent to 250 mg ampicillin. 100 mL bottles NDC 67253-183-10 200 mL bottles NDC 67253-183-20 Store dry powder at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Store the reconstituted suspension in a refrigerator. Discard any unused portion after 14 days. Manufactured for: DAVA Pharmaceuticals, Inc . Fort Lee, NJ 07024, USA by: STADA Production Ireland Ltd. Clonmel, Ireland. Rev. 01/11 181G451
Indications & Usage
INDICATIONS AND USAGE To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin capsules, Ampicillin for Oral Suspension and other antibacterial drugs, Ampicillin capsules and Ampicillin for Oral Suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting of modifying anitimicrobial therapy, in the absence of such data, local epidemiology and susceptibility patterns contribute to the empiric selection of therapy. Ampicillin capsules and Ampicillin for oral suspension are indicated in the treatment of infections caused by susceptible strains of the designated organisms listed below: Infections of the genitourinary tract including gonorrhea - E. coli, P. mirabilis , enterococci, Shigella , S. typhosa and other Salmonella and nonpenicillinase-producing N. gonorrhoeae . Infections of the respiratory tract - Nonpenicillinase-producing H. influenzae and staphylococci, and streptococci including Streptococcus pneumoniae . Infections of the gastrointestinal tract - Shigella , S. typhosa and other Salmonella, E. coli, P. mirabilis , and enterococci. Meningitis - N. Meningitidis Bacteriology studies to determine the causative organisms and their susceptibility to ampicillin should be performed. Therapy may be instituted prior to the results of susceptibility testing.
Dosage and Administration
DOSAGE AND ADMINISTRATION Adults and children weighing over 20 Kg: For genitourinary or gastrointestinal tract infections other than gonorrhea in men and women , the usual dose is 500 mg q.i.d. in equally spaced doses; severe or chronic infections may require larger doses. For the treatment of gonorrhea in both men and women, a single oral dose of 3.5 grams of ampicillin administered simultaneously with 1 gram of probenecid is recommended. Physicians are cautioned to use no less than the above recommended dosage for the treatment of gonorrhea. Follow-up cultures should be obtained from the original site(s) of infection 7 to 14 days after therapy. In women, it is also desirable to obtain culture test-of-cure from both the endocervical and anal canals. Prolonged intensive therapy is needed for complications such as prostatitis and epididymitis. For respiratory tract infections , the usual dose is 250 mg q.i.d. in equally spaced doses. Pediatric Patients weighing 20 Kg or less: For genitourinary or gastrointestinal tract infections , the usual dose is 100 mg/kg/day total, q.i.d. in equally divided and spaced doses. For respiratory tract infections , the usual dose is 50 mg/kg/day total, in equally divided and spaced doses three to four times daily. Doses for children should not exceed doses recommended for adults. All patients, irrespective of age and weight: Larger doses may be required for severe or chronic infections. Although ampicillin is resistant to degradation by gastric acid, it should be administered at least one half-hour before or two hours after meals for maximal absorption. Except for the single dose regimen for gonorrhea referred to above, therapy should be continued for a minimum of 48 to 72 hours after the patient becomes asymptomatic or evidence at bacterial eradication has been obtained. In infections caused by haemolytic strains of streptococci, a minimum of 10 days' treatment is recommended to guard against the risk of rheumatic fever or glomerulonephritis (see PRECAUTIONS , Laboratory Tests ). In the treatment of chronic urinary or gastrointestinal infections, frequent bacteriologic and clinical appraisal is necessary during therapy and may be necessary for several months afterwards. Stubborn infections may require treatment for several weeks. Smaller doses than those indicated above should not be used. Directions for mixing Oral Suspension Prepare suspension at time of dispensing. For ease of preparation, add water to the bottle in two portions and shake well after each addition. 125 mg/5 mL Add a total of 86 mL to the 100 mL package and 170 mL to the 200 mL package. This will provide 100 mL and 200 mL of suspension. Each 5 mL (teaspoonful) will contain ampicillin trihydrate equivalent to 125 mg ampicillin. 250 mg/5 mL Add a total of 70 mL to the 100 mL package and 139 mL to the 200 mL package. This will provide 100 mL and 200 mL of suspension . Each 5 mL (teaspoonful) will contain ampicillin trihydrate equivalent to 250 mg ampicillin.
