Drug Catalog - Product Detail
AMOXICILLIN & CLAVULANATE POTASSIUM FOR SUSPENSION 600/42.9MG/5ML 200ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00143-9853-24 | HIKMA PHARMACEUTICALS USA | 200 | 600-42.9MG/5ML | SUSPENSION |
PACKAGE FILES



Generic Name
AMOXICILLIN AND CLAVULANATE POTASSIUM
Substance Name
AMOXICILLIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA065373
Description
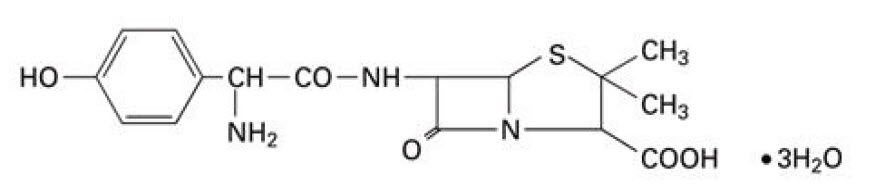
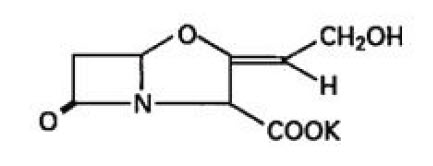
11 DESCRIPTION Amoxicillin and Clavulanate Potassium for Oral Suspension USP, 600 mg/42.9 mg per 5 mL is an oral antibacterial combination consisting of the semisynthetic antibacterial amoxicillin and the beta-lactamase inhibitor, clavulanate potassium (the potassium salt of clavulanic acid). Amoxicillin is an analog of ampicillin, derived from the basic penicillin nucleus, 6-aminopenicillanic acid. The amoxicillin molecular formula is C 16 H 19 N 3 O 5 S•3H 2 O, and the molecular weight is 419.46. Chemically, amoxicillin is (2 S ,5 R ,6 R )-6-[(R)-(-)-2-Amino-2-( p -hydroxyphenyl) acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid trihydrate and may be represented structurally as: Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus . It is a beta-lactam structurally related to the penicillins and possesses the ability to inactivate a wide variety of beta-lactamases by blocking the active sites of these enzymes. Clavulanic acid is particularly active against the clinically important plasmid-mediated beta-lactamases frequently responsible for transferred drug resistance to penicillins and cephalosporins. The clavulanate potassium molecular formula is C 8 H 8 KNO 5 and the molecular weight is 237.25. Chemically, clavulanate potassium is potassium ( Z )-(2 R ,5 R )-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]-heptane-2-carboxylate and may be represented structurally as: Following constitution, each 5 mL of oral suspension contains 600 mg of amoxicillin as the trihydrate and 42.9 mg of clavulanic acid (equivalent to 51.1 mg of clavulanate potassium). Potassium content 0.248 mEq per 5 mL Inactive Ingredients: Aspartame, colloidal silicon dioxide, hypromellose, orange powder flavor, silicon dioxide, succinic acid, xanthan gum [see Warnings and Precautions ( 5.8 )] . Each 5 mL of reconstituted amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL contains approximately 9.73 mg of potassium. amoxicillin-structural-formula clavulanic-acid-structural-formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied Amoxicillin and Clavulanate Potassium for Oral Suspension USP, 600 mg/42.9 mg per 5 mL: Each 5 mL of reconstituted orange-flavored suspension contains 600 mg of amoxicillin as the trihydrate and 42.9 mg clavulanic acid as the potassium salt (equivalent to 51.1 mg of clavulanate potassium). NDC 0143-9853-75 75 mL bottle NDC 0143-9853-16 125 mL bottle NDC 0143-9853-24 200 mL bottle Storage Store reconstituted suspension under refrigeration. Discard unused suspension after 10 days. Store dry powder at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature. Dispense in original container].
Indications & Usage
1 INDICATIONS AND USAGE Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is indicated for the treatment of pediatric patients aged 3 months to 12 years weighing less than or equal to 40 kg with: Recurrent or persistent acute otitis media due to S. pneumoniae (penicillin MICs less than or equal to 2 mcg/mL), H. influenzae (including beta-lactamase-producing strains), or M. catarrhalis (including beta-lactamase-producing strains) characterized by the following risk factors: -Antibacterial drug exposure for acute otitis media within the preceding 3 months, and either of the following: 1) age 2 years, or younger or 2) day care attendance [see Microbiology ( 12.4 )] . Limitations of Use Acute otitis media due to S. pneumoniae alone can be treated with amoxicillin. Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is not indicated for the treatment of acute otitis media due to S. pneumoniae with penicillin MIC greater than or equal to 4 mcg/mL. Therapy may be instituted prior to obtaining the results from bacteriological studies when there is reason to believe the infection may involve both S. pneumoniae (penicillin MIC less than or equal to 2 mcg/mL) and the beta-lactamase-producing organisms listed above. Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL and other antibacterial drugs, Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL is a combination of amoxicillin, a penicillin-class antibacterial and clavulanate potassium, a beta-lactamase inhibitor, indicated for the treatment of pediatric patients aged 3 months to 12 years weighing less than or equal to 40 kg with Recurrent or persistent acute otitis media due to S. pneumoniae (penicillin MICs less than or equal to 2 mcg/mL), H. influenzae (including beta-lactamase-producing strains), or M. catarrhalis (including beta-lactamase-producing strains) characterized by the following risk factors ( 1 ): - Antibacterial exposure for acute otitis media within the preceding 3 months, and either of the following: 1) age 2 years, or younger or 2) daycare attendance. Limitations of Use Acute otitis media due to S. pneumoniae alone can be treated with amoxicillin. Amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL is not indicated for the treatment of acute otitis media due to S. pneumoniae with penicillin MIC greater than or equal to 4 mcg/mL. Therapy may be instituted prior to obtaining the results from bacteriological studies when there is reason to believe the infection may involve both S. pneumoniae (penicillin MIC less than or equal to 2 mcg/mL) and the beta-lactamase-producing organisms listed above. ( 1 ) Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of Amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL and other antibacterial drugs, Amoxicillin and clavulanate potassium for oral suspension, 600 mg/42.9 mg per 5 mL should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Pediatric Patients aged 3 months to 12 years weighing less than or equal to 40 kg: 90 mg/kg/day divided every 12 hours, administered for 10 days. ( 2 ) 2.1 Important Administration Instructions To minimize the potential for gastrointestinal intolerance, amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL should be taken at the start of a meal. Absorption of clavulanate potassium may be enhanced when amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is administered at the start of a meal. 2.2 Dosage in Pediatric Patients Pediatric patients aged 3 months to 12 years weighing less than or equal to 40 kg: Based on the amoxicillin component (600 mg/5 mL), the recommended dose of amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL is 90 mg/kg/day divided every 12 hours, administered for 10 days (see Table 1 as a general example guideline for attainment of this dosage). This dose provides 6.4 mg/kg/day of the clavulanic acid component. Table 1: General Dosage Guidelines for Amoxicillin and Clavulanate Potassium for Oral Suspension 600 mg/42.9 mg per 5 mL in Pediatric Patients Body Weight (kg) Volume of Amoxicillin and Clavulanate Potassium for Oral Suspension 600 mg/42.9 mg per 5 mL Providing 90 mg/kg/day 8 3 mL twice daily 12 4.5 mL twice daily 16 6 mL twice daily 20 7.5 mL twice daily 24 9 mL twice daily 28 10.5 mL twice daily 32 12 mL twice daily 36 13.5 mL twice daily 40 15 mL twice daily Pediatric patients weighing greater than 40 kg : Experience with amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL in this group is not available. 2.3 Dosage in Adult Patients Experience with amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL in adults is not available and adults who have difficulty swallowing should not be given amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL in place of the 500 mg or 875 mg tablet of amoxicillin and clavulanate potassium. 2.4 Dosage in Patients with Hepatic Impairment Hepatically impaired patients should be dosed with caution and hepatic function monitored at regular intervals [see Warnings and Precautions ( 5.4 )] . 2.5 Preparation of the Oral Suspension Prepare the suspension at time of dispensing as follows: Tap bottle until all powder flows freely. Measure the total amount of water (see Table 2) to be added in two parts. Add approximately 2/3 of the total amount of water for reconstitution, replace cap and shake vigorously to suspend powder. Add remainder of the water (that had been measured), replace cap and again shake vigorously. Table 2: Volume of Water for Reconstituting Amoxicillin and Clavulanate Potassium for Oral Suspension 600 mg/42.9 mg per 5 mL Bottle Size Amount of Water Required for Reconstitution 75 mL 66 mL 125 mL 110 mL 200 mL 176 mL Each 5 mL will contain 600 mg of amoxicillin as the trihydrate, and 42.9 mg of clavulanic acid as the potassium salt. Shake oral suspension well before each use. Suspension must be refrigerated. Discard after 10 days. Suspension is off-white at time of reconstitution; some color change is normal during the dosing period. Flavoring Information: For patients who wish to alter the taste of amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL, immediately after reconstitution, 1 drop of FLAVORx ® (apple, banana cream, bubble gum, cherry, or watermelon flavor) may be added for every 5 mL of amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL. The resulting suspension is stable for 10 days under refrigeration. Stability of amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL when mixed with other flavors other than the 5 flavors listed above has not been evaluated. 2.6 Switching between Dosage Forms and between Strengths Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL does not contain the same amount of clavulanic acid (as the potassium salt) as any of the other suspensions of amoxicillin and clavulanate potassium for oral suspension. Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL contains 42.9 mg of clavulanic acid per 5 mL, whereas the 200 mg/28.5 mg per 5 mL suspension of Amoxicillin and clavulanate potassium contains 28.5 mg clavulanic acid per 5 mL and the 400 mg/57 mg per 5 mL suspension of Amoxicillin and clavulanate potassium contains 57 mg clavulanic acid per 5 mL. Therefore, the 200 mg/28.5 mg per 5 mL and 400 mg/57 mg per 5 mL suspensions of Amoxicillin and clavulanate potassium should not be substituted for Amoxicillin and clavulanate potassium for oral suspension 600 mg/42.9 mg per 5 mL as they are not interchangeable.
