Drug Catalog - Product Detail
AMOXI & CLAV POT. TAB 875/125 MG 20CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00143-9249-20 | HIKMA PHARMACEUTICALS USA | 20 | 875-125MG | TABLET |
PACKAGE FILES
Generic Name
AMOXICILLIN AND CLAVULANATE POTASSIUM
Substance Name
AMOXICILLIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203824
Description
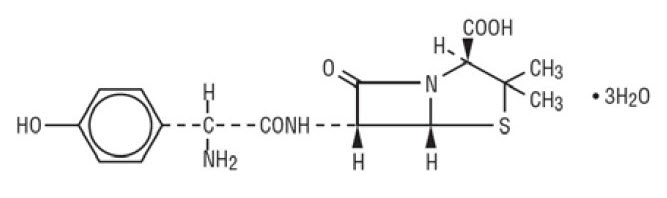
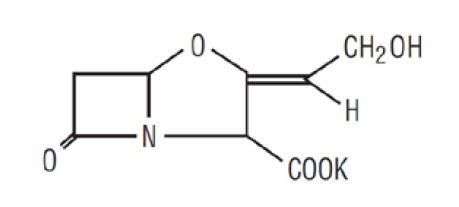
11 DESCRIPTION Amoxicillin and Clavulanate Potassium Tablets, USP are an oral antibacterial combination consisting of amoxicillin and the beta-lactamase inhibitor, clavulanate potassium (the potassium salt of clavulanic acid). Amoxicillin is an analog of ampicillin, derived from the basic penicillin nucleus, 6-aminopenicillanic acid. The amoxicillin molecular formula is C 16 H 19 N 3 O 5 S•3H 2 O, and the molecular weight is 419.46. Chemically, amoxicillin is (2 S ,5 R ,6 R )-6-[( R )-(-)-2-Amino-2-( p -hydroxyphenyl) acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate and may be represented structurally as: Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus . It is a beta-lactam structurally related to the penicillins and possesses the ability to inactivate some beta-lactamases by blocking the active sites of these enzymes. The clavulanate potassium molecular formula is C 8 H 8 KNO 5 , and the molecular weight is 237.25. Chemically, clavulanate potassium is potassium ( Z )( 2R , 5R )-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]-heptane-2-carboxylate and may be represented structurally as: Inactive Ingredients: Colloidal silicon dioxide, ethylcellulose, hypromellose, magnesium stearate, microcrystalline cellulose, propylene glycol, sodium starch glycolate, and titanium dioxide. Each tablet of amoxicillin/clavulanate potassium contains 0.63 mEq potassium. FDA approved dissolution test specifications differ from USP. amoxicillin structural formula clavulanate structural formula
How Supplied
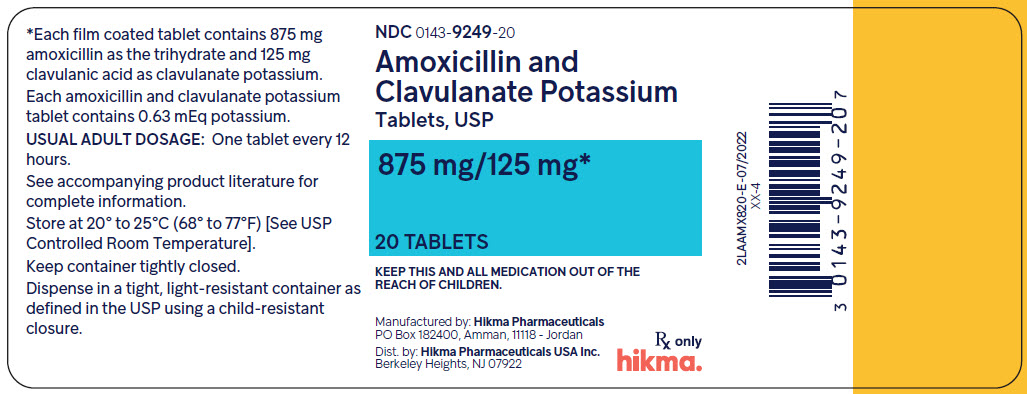
16 HOW SUPPLIED/STORAGE AND HANDLING Amoxicillin and Clavulanate Potassium Tablets USP, 875mg/125mg: Each scored Off-white film coated capsule-shaped tablet, debossed with WW949 on the upper side and bisected on the other side, contains 875 mg amoxicillin as the trihydrate and 125 mg clavulanic acid as the potassium salt (equivalent to 149 mg of clavulanate potassium). NDC 0143-9249-20 ........... 20 tablets bottle Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP, with a child-resistant closure (as required). Advise patients to keep in a closed container. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Indications & Usage
1 INDICATIONS AND USAGE Amoxicillin and Clavulanate Potassium Tablets are indicated for the treatment of infections in adults and pediatric patients, due to susceptible isolates of the designated bacteria in the conditions listed below: Lower Respiratory Tract Infections -caused by beta‑lactamase‑producing isolates of Haemophilus influenzae and Moraxella catarrhalis . Acute Bacterial Otitis Media -caused by beta‑lactamase‑producing isolates of H. influenzae and M. catarrhalis . Sinusitis -caused by beta‑lactamase‑producing isolates of H. influenzae and M. catarrhalis . Skin and Skin Structure Infections -caused by beta‑lactamase‑producing isolates of Staphylococcus aureus , Escherichia coli , and Klebsiella species. Urinary Tract Infections -caused by beta‑lactamase‑producing isolates of E. coli , Klebsiella species, and Enterobacter species. Limitations of Use When susceptibility test results show susceptibility to amoxicillin, indicating no beta-lactamase production, Amoxicillin and Clavulanate Potassium Tablets should not be used. Usage To reduce the development of drug‑resistant bacteria and maintain the effectiveness of Amoxicillin and Clavulanate Potassium Tablets and other antibacterial drugs, Amoxicillin and Clavulanate Potassium Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Amoxicillin and Clavulanate Potassium Tablets are a combination of amoxicillin, a penicillin-class antibacterial and clavulanate potassium, a beta‑lactamase inhibitor indicated for treatment of the following infections in adults and pediatric patients: ( 1 ) Lower respiratory tract infections Acute bacterial otitis media Sinusitis Skin and skin structure infections Urinary tract infections Limitations of Use When susceptibility test results show susceptibility to amoxicillin, indicating no beta-lactamase production, Amoxicillin and Clavulanate Potassium Tablets should not be used. ( 1 ) Usage To reduce the development of drug-resistant bacteria and maintain the effectiveness of Amoxicillin and Clavulanate Potassium Tablets and other antibacterial drugs, Amoxicillin and Clavulanate Potassium Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Adults and Pediatric Patients greater than 40 kg: 875 mg every 12 hours, based on amoxicillin component. ( 2.2 , 2.3 ) 2.1 Important Administration Instructions Amoxicillin and Clavulanate Potassium Tablets may be taken without regard to meals; however, absorption of clavulanate potassium is enhanced when Amoxicillin and Clavulanate Potassium Tablets are administered at the start of a meal. To minimize the potential for gastrointestinal intolerance, Amoxicillin and Clavulanate Potassium Tablets should be taken at the start of a meal. 2.2 Adult Patients See dosing regimens of Amoxicillin and Clavulanate Potassium Tablets (based on the amoxicillin component) provided in Table 1 below. Table 1. Dosing Regimens of Amoxicillin and Clavulanate Potassium Tablets in Adult Patients TYPE OF INFECTION DOSING REGIMEN OF AMOXICILLIN AND CLAVULANATE POTASSIUM TABLETS Severe infections and infections of the respiratory tract one 875 mg tablet a of Amoxicillin and Clavulanate Potassium every 12 hours or one 500 mg tablet b,c of Amoxicillin and Clavulanate Potassium every 8 hours Less severe infections one 500 mg tablet b,c of Amoxicillin and Clavulanate Potassium every 12 hours or one 250 mg tablet d of Amoxicillin and Clavulanate Potassium every 8 hours a Adults who have difficulty swallowing may be given the Amoxicillin and Clavulanate Potassium 200 mg/28.5 mg per 5 mL suspension or the Amoxicillin and Clavulanate Potassium 400 mg/57 mg per 5 mL suspension may be used in place of the 875 mg/125 mg tablet. b Adults who have difficulty swallowing may be given the Amoxicillin and Clavulanate Potassium 125 mg/31.25 mg per 5 mL or Amoxicillin and Clavulanate Potassium 250 mg/62.5 mg per 5 mL suspension in place of the 500 mg/125 mg tablet. c Two Amoxicillin and Clavulanate Potassium 250 mg/125 mg tablets are NOT substitutable with one 500 mg/125 mg Amoxicillin and Clavulanate Potassium tablet [see Dosage and Administration (2.6)] . d Amoxicillin and Clavulanate Potassium 250 mg/125 mg tablet is NOT substitutable with Amoxicillin and Clavulanate Potassium 250 mg/62.5 mg chewable tablet [see Dosage and Administration (2.6)] . 2.3 Pediatric Patients Based on the amoxicillin component, Amoxicillin and Clavulanate Potassium should be dosed as follows: Neonates and Infants Aged less than 12 weeks (less than 3 months): See dosing regimens of Amoxicillin and Clavulanate Potassium provided in Table 2 below. Table 2: Dosing Regimens of Amoxicillin and Clavulanate Potassium in Neonates and Infants Aged Less than 12 Weeks (Less than 3 Months) PATIENT POPULATION DOSING REGIMEN Amoxicillin and Clavulanate Potassium 125 mg/31.25 mg per 5 mL for oral suspension a Neonates and Infants aged less than 12 weeks (less than 3 months) 30 mg/kg/day every 12 hours a Experience with the Amoxicillin and Clavulanate Potassium 200 mg/28.5 mg per 5 mL formulation in this age group is limited, and thus, use of the Amoxicillin and Clavulanate Potassium 125 mg/31.25 mg per 5 mL for oral suspension is recommended. Patients Weighing 40 kg or More : Pediatric patients weighing 40 kg or more should be dosed according to adult recommendations. The 250 mg/125 mg tablet of Amoxicillin and Clavulanate Potassium should NOT be used until the child weighs at least 40 kg, due to the different amoxicillin to clavulanic acid ratios in the 250 mg/125 mg tablet of Amoxicillin and Clavulanate Potassium versus the 250 mg/62.5 mg chewable tablet of Amoxicillin and Clavulanate Potassium. 2.4 Patients with Renal Impairment Patients with impaired renal function do not generally require a reduction in dose unless the impairment is severe. Renal impairment patients with a glomerular filtration rate (GFR) of less than 30 mL/min should NOT receive the 875 mg dose (based on the amoxicillin component) of Amoxicillin and Clavulanate Potassium Tablets.
