Drug Catalog - Product Detail
AMLODIPINE-OLMESARTAN ORAL TABLET 10-40MG 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 33342-0193-07 | MACLEODS PHARMACEUTICALS | 30 | 10-40MG | NA |
PACKAGE FILES








Generic Name
AMLODIPINE AND OLMESARTAN MEDOXOMIL
Substance Name
AMLODIPINE BESYLATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA206884
Description
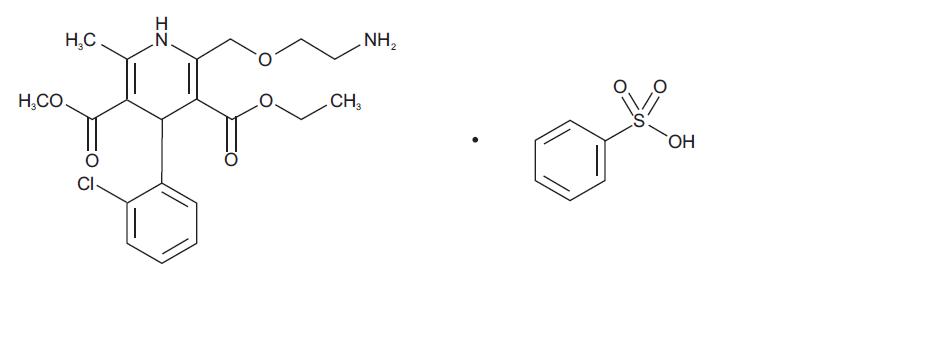
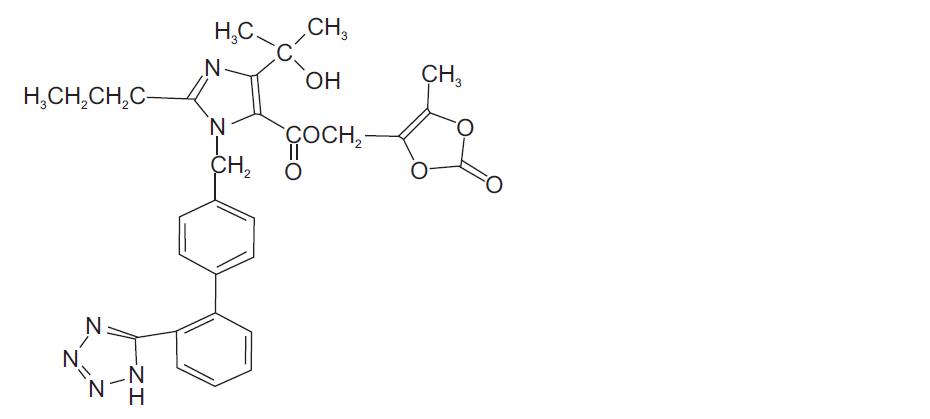
11 DESCRIPTION Amlodipine and olmesartan medoxomil provided as a tablet for oral administration, is a combination of the calcium channel blocker (CCB) amlodipine besylate and the angiotensin II receptor blocker (ARB) olmesartan medoxomil. The amlodipine besylate component, USP of amlodipine and olmesartan medoxomil tablets is chemically described as 3-ethyl-5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its empirical formula is C 20 H 25 ClN 2 O 5 •C 6 H 6 O 3 S. Olmesartan medoxomil, USP a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. The olmesartan medoxomil, USP component of amlodipine and olmesartan medoxomil tablets is chemically described as 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[ p -(o-1 H -tetrazol-5-ylphenyl)benzyl]imidazole-5-carboxylate, cyclic 2,3-carbonate. Its empirical formula is C 29 H 30 N 6 O 6 . The structural formula for amlodipine besylate, USP is: The structural formula for olmesartan medoxomil, USP is: Amlodipine and olmesartan medoxomil tablets contains amlodipine besylate, USP a white to off-white crystalline powder, and olmesartan medoxomil, USP a white to light yellowish-white powder or crystalline powder. The molecular weights of amlodipine besylate and olmesartan medoxomil are 567.1 and 558.59, respectively. Amlodipine besylate, USP is slightly soluble in water and sparingly soluble in ethanol. Olmesartan medoxomil, USP is practically insoluble in water and sparingly soluble in methanol. Each tablet of amlodipine and olmesartan medoxomil tablets also contains the following inactive ingredients: silicified microcrystalline cellulose, colloidal silicon dioxide, pregelatinized maize starch, croscarmellose sodium, and magnesium stearate. The color coatings contain polyvinyl alcohol, macrogol/ polyethylene glycol 3350, titanium dioxide, talc, iron oxide yellow (5 mg/40 mg, 10 mg/20 mg, 10 mg/40 mg tablets), iron oxide red (10 mg/20 mg and 10 mg/40 mg tablets). amlo olme
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Amlodipine and olmesartan medoxomil tablets contain amlodipine besylate at a dose equivalent to 5 or 10 mg amlodipine and olmesartan medoxomil in the strengths described below. Amlodipine and olmesartan medoxomil tablets are differentiated by tablet color/size and are debossed with an individual product tablet code on one side. Amlodipine and olmesartan medoxomil tablets are supplied for oral administration in the following strength and package configurations: Tablet Strength (amlodipine equivalent in mg/ olmesartan medoxomil in mg) Package Configuration NDC# Product Code Tablet Color 5 mg/20 mg Bottle of 30 Bottle of 90 10 blisters of 10 Bottle of 1000 33342-190-07 33342-190-10 33342-190-12 33342-190-44 L75 White 10 mg/20 mg Bottle of 30 Bottle of 90 10 blisters of 10 Bottle of 1000 33342-192-07 33342-192-10 33342-192-12 33342-192-44 L76 Grayish Orange 5 mg/40 mg Bottle of 30 Bottle of 90 10 blisters of 10 Bottle of 1000 33342-191-07 33342-191-10 33342-191-12 33342-191-44 L77 Light Yellow 10 mg/ 40 mg Bottle of 30 Bottle of 90 10 blisters of 10 Bottle of 1000 33342-193-07 33342-193-10 33342-193-12 33342-193-44 L78 Brownish Red Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
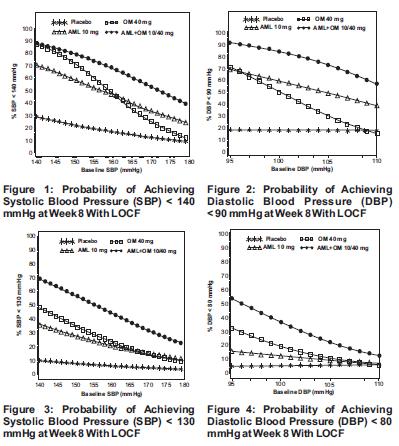
1 INDICATIONS & USAGE Amlodipine and olmesartan medoxomil tablets are indicated for the treatment of hypertension, alone or with other antihypertensive agents, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular (CV) events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with amlodipine and olmesartan medoxomil tablets. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Amlodipine and olmesartan medoxomil tablets may also be used as initial therapy in patients who are likely to need multiple antihypertensive agents to achieve their blood pressure goals. Patients with moderate or severe hypertension are at relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and should be shaped by considerations such as baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared to monotherapy. Individual blood pressure goals may vary based upon the patient’s risk. Data from an 8-week, placebo-controlled, parallel-group factorial study [see Clinical Studies ( 14.1 )] provide estimates of the probability of reaching a blood pressure goal with amlodipine and olmesartan medoxomil tablets compared to amlodipine or olmesartan medoxomil monotherapy. The figures below provide estimates of the likelihood of achieving the targeted systolic or diastolic blood pressure goals with amlodipine and olmesartan medoxomil tablets 10 mg/40 mg compared with amlodipine or olmesartan medoxomil monotherapy, based upon baseline systolic or diastolic blood pressure. The curve of each treatment group was estimated by logistic regression modeling from all available data of that treatment group. The right tail of each curve is less reliable because of small numbers of subjects with high baseline blood pressures. The figures above provide an approximation of the likelihood of reaching a targeted blood pressure goal (e.g., Week 8 SBP <140 mmHg or <130 mmHg or a DBP <90 mmHg or <80 mmHg) for the high-dose treatment groups evaluated in the study. Amlodipine and olmesartan medoxomil tablets 5 mg/20 mg, the lowest dose combination treatment group, increases the probability of reaching blood pressure goal compared with the highest dose monotherapies, amlodipine 10 mg and olmesartan medoxomil 40 mg. For example, a patient with a baseline blood pressure of 160/100 mmHg has about a 48% likelihood of achieving a goal of <140 mmHg (systolic) and a 51% likelihood of achieving a goal of <90 mmHg (diastolic) on monotherapy with olmesartan medoxomil 40 mg, and about a 46% likelihood of achieving a goal of <140 mmHg (systolic) and a 60% likelihood of achieving a goal of <90 mmHg (diastolic) on monotherapy with amlodipine 10 mg. The likelihood of achieving these same goals increases to 63% (systolic) and 71% (diastolic) on amlodipine and olmesartan medoxomil tablets 5 mg/20 mg, and to 68% (systolic) and 85% (diastolic) on amlodipine and olmesartan medoxomil tablets 10 mg/40 mg. • Amlodipine and olmesartan medoxomil tablets are a combination of amlodipine besylate, a dihydropyridine calcium channel blocker and olmesartan medoxomil, an angiotensin II receptor blocker, indicated for the treatment of hypertension, alone or with other antihypertensive agents, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. ( 1 ). • Amlodipine and olmesartan medoxomil tablets may also be used as initial therapy in patients likely to need multiple antihypertensive agents to achieve their blood pressure goals ( 1 ). taba
Dosage and Administration
2 DOSAGE & ADMINISTRATION The usual starting dose of amlodipine and olmesartan medoxomil tablets is 5/20 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum dose of one 10/40 mg tablet once daily as needed to control blood pressure [see Clinical Studies ( 14.1 )]. • Recommended starting dose: 5 mg/20 mg once daily ( 2 ). • Titrate as needed in two week intervals up to a maximum of 10 mg/40 mg once daily ( 2 ).
