Drug Catalog - Product Detail
AMLODIPINE BESYLATE/ATORVASTATIN CALCIUM TB 2.5/10MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43598-0323-30 | DR.REDDY'S LABORATORIES, INC. | 30 | 2.5-10MG | TABLET |
PACKAGE FILES











Generic Name
AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Substance Name
AMLODIPINE BESYLATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203874
Description
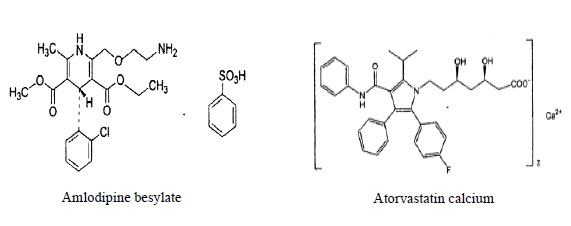
11 DESCRIPTION Amlodipine besylate and atorvastatin calcium tablets combine the calcium channel blocker amlodipine besylate USP with the HMG CoA-reductase inhibitor atorvastatin calcium. The amlodipine besylate USP is chemically described as 3-ethyl-5-methyl (4RS)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzenesulphonate. Its molecular formula is C 20 H 25 ClN 2 O 5 •C 6 H 6 O 3 S. The atorvastatin calcium is chemically described as [R-(R*, R*)]-2-(4-fluorophenyl)-ß, δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1). Its molecular formula is C 66 H 68 CaF 2 N 4 O 10 . The structural formulae for amlodipine besylate USP and atorvastatin calcium are shown below. Amlodipine besylate and atorvastatin calcium contains amlodipine besylate USP, a white or almost white powder, and atorvastatin calcium, a white to off-white colored powder, free from visible extraneous matter. Amlodipine besylate USP has a molecular weight of 567.1 and atorvastatin calcium has a molecular weight of 1155.36. Amlodipine besylate USP is freely soluble in methanol, sparingly soluble in ethanol, slightly soluble in water and in 2-proponal. Atorvastatin calcium is soluble in dimethyl sulphoxide, slightly soluble in alcohol, very slightly soluble in water, in pH 7.4 phosphate buffer and in acetonitrile and practically insoluble in aqueous solutions of pH 4 and below. Amlodipine besylate and atorvastatin calcium is available as film-coated tablets containing: 2.5 mg amlodipine equivalent to 3.468 mg amlodipine besylate USP and 10 mg atorvastatin equivalent to 10.341 mg atorvastatin calcium. 2.5 mg amlodipine equivalent to 3.468 mg amlodipine besylate USP and 20 mg atorvastatin equivalent to 20.683 mg atorvastatin calcium. 2.5 mg amlodipine equivalent to 3.468 mg amlodipine besylate USP and 40 mg atorvastatin equivalent to 41.365 mg atorvastatin calcium. 5 mg amlodipine equivalent to 6.935 mg amlodipine besylate USP and 10 mg atorvastatin equivalent to 10.341 mg atorvastatin calcium. 5 mg amlodipine equivalent to 6.935 mg amlodipine besylate USP and 20 mg atorvastatin equivalent to 20.683 mg atorvastatin calcium. 5 mg amlodipine equivalent to 6.935 mg amlodipine besylate USP and 40 mg atorvastatin equivalent to 41.365 mg atorvastatin calcium. 5 mg amlodipine equivalent to 6.935 mg amlodipine besylate USP and 80 mg atorvastatin equivalent to 82.730 mg atorvastatin calcium. 10 mg amlodipine equivalent to 13.870 mg amlodipine besylate USP and 10 mg atorvastatin equivalent to 10.341 mg atorvastatin calcium. 10 mg amlodipine equivalent to 13.870 mg amlodipine besylate USP and 20 mg atorvastatin equivalent to 20.683 mg atorvastatin calcium. 10 mg amlodipine equivalent to 13.870 mg amlodipine besylate USP and 40 mg atorvastatin equivalent to 41.365 mg atorvastatin calcium. 10 mg amlodipine equivalent to 13.870 mg amlodipine besylate USP and 80 mg atorvastatin equivalent to 82.730 mg atorvastatin calcium. Each film-coated tablet also contains colloidal silicon dioxide, crospovidone, hydroxy propyl cellulose, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium bicarbonate, sodium lauryl sulfate, Opadry- II White 85G18490 (Polyethylene glycol, polyvinyl alcohol, lecithin, talc and titanium dioxide), or Opadry- II Blue 85G50642 (FD&C Blue No.2, polyethylene glycol, polyvinyl alcohol, lecithin, talc and titanium dioxide).
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Amlodipine besylate and atorvastatin calcium tablets contain amlodipine besylate USP and atorvastatin calcium equivalent to amlodipine and atorvastatin in the dose strengths described below. Amlodipine besylate and atorvastatin calcium tablets 2.5 mg/10 mg are white to off-white, round, film coated tablets debossed ‘R’ on one side and ‘407’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-323-30 Bottles of 60 NDC 43598-323-60 Bottles of 90 NDC 43598-323-90 Bottles of 500 NDC 43598-323-05 Amlodipine besylate and atorvastatin calcium tablets 2.5 mg/20 mg are white to off-white, round, film coated tablets debossed ‘R’ on one side and ‘408’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-320-30 Bottles of 60 NDC 43598-320-60 Bottles of 90 NDC 43598-320-90 Bottles of 500 NDC 43598-320-05 Amlodipine besylate and atorvastatin calcium tablets 2.5 mg/40 mg are white to off-white, round, film coated tablets debossed ‘R’ on one side and ‘409’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-317-30 Bottles of 60 NDC 43598-317-60 Bottles of 90 NDC 43598-317-90 Bottles of 500 NDC 43598-317-05 Amlodipine besylate and atorvastatin calcium tablets 5 mg/10 mg are white to off-white, oval shaped, film coated tablets debossed ‘R’ on one side and ‘410’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-322-30 Bottles of 60 NDC 43598-322-60 Bottles of 90 NDC 43598-322-90 Bottles of 500 NDC 43598-322-05 Amlodipine besylate and atorvastatin calcium tablets 5 mg/20 mg are white to off-white, oval shaped, film coated tablets debossed ‘R’ on one side and ‘411’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-319-30 Bottles of 60 NDC 43598-319-60 Bottles of 90 NDC 43598-319-90 Bottles of 500 NDC 43598-319-05 Amlodipine besylate and atorvastatin calcium tablets 5 mg/40 mg are white to off-white, oval shaped, film coated tablets debossed ‘R’ on one side and ‘412’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-316-30 Bottles of 60 NDC 43598-316-60 Bottles of 90 NDC 43598-316-90 Bottles of 500 NDC 43598-316-05 Amlodipine besylate and atorvastatin calcium tablets 5 mg/80 mg are white to off-white, oval shaped, film coated tablets debossed ‘R’ on one side and ‘413’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-314-30 Bottles of 60 NDC 43598-314-60 Bottles of 90 NDC 43598-314-90 Bottles of 500 NDC 43598-314-05 Amlodipine besylate and atorvastatin calcium tablets 10 mg/10 mg are blue, oval shaped, film coated tablets debossed ‘R’ on one side and ‘414’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-321-30 Bottles of 60 NDC 43598-321-60 Bottles of 90 NDC 43598-321-90 Bottles of 500 NDC 43598-321-05 Amlodipine besylate and atorvastatin calcium tablets 10 mg/20 mg are blue, oval shaped, film coated tablets debossed ‘R’ on one side and ‘415’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-318-30 Bottles of 60 NDC 43598-318-60 Bottles of 90 NDC 43598-318-90 Bottles of 500 NDC 43598-318-05 Amlodipine besylate and atorvastatin calcium tablets 10 mg/40 mg are blue, oval shaped, film coated tablets debossed ‘R’ on one side and ‘416’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-315-30 Bottles of 60 NDC 43598-315-60 Bottles of 90 NDC 43598-315-90 Bottles of 500 NDC 43598-315-05 Amlodipine besylate and atorvastatin calcium tablets 10 mg/80 mg are blue, oval shaped, film coated tablets debossed ‘R’ on one side and ‘417’ on other side and are supplied in bottles of 30’s, 60’s, 90’s and 500’s. Bottles of 30 NDC 43598-313-30 Bottles of 60 NDC 43598-313-60 Bottles of 90 NDC 43598-313-90 Bottles of 500 NDC 43598-313-05 Store Amlodipine besylate and atorvastatin calcium tablets at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Indications & Usage
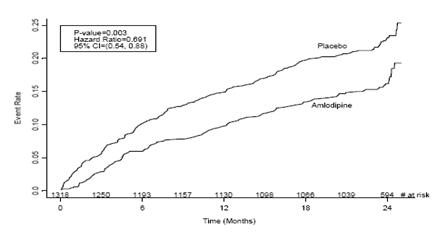
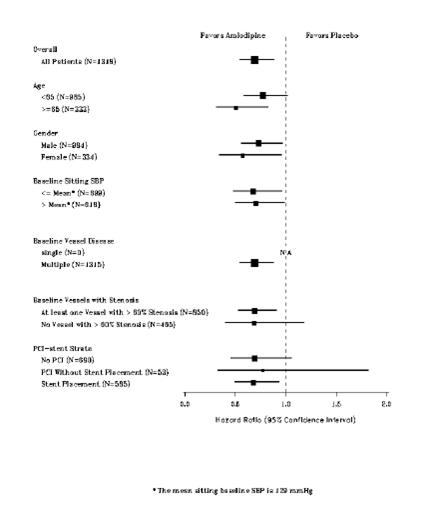
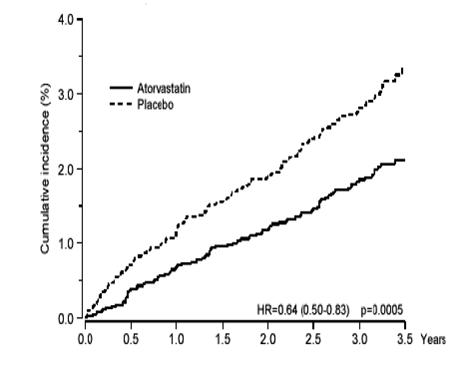
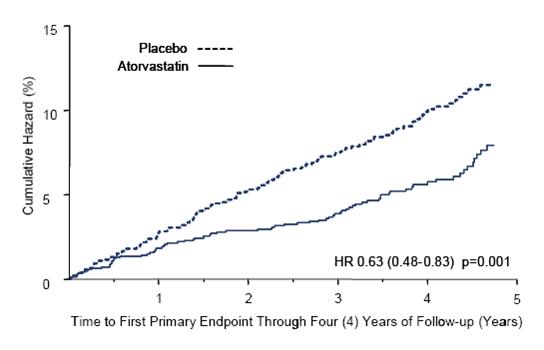
1 INDICATIONS AND USAGE Amlodipine besylate and atorvastatin calcium tablets are indicated in patients for whom treatment with both amlodipine and atorvastatin is appropriate. Amlodipine Amlodipine besylate and atorvastatin calcium tablets are a combination of amlodipine besylate, a calcium channel blocker, and atorvastatin calcium, a HMG CoA-reductase inhibitor, indicated in patients for whom treatment with both amlodipine and atorvastatin is appropriate. Amlodipine is indicated for the treatment of hypertension, to lower blood pressure (1.1). Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. Amlodipine is indicated for the treatment of Coronary Artery Disease (1.2). Atorvastatin is indicated as an adjunct therapy to diet for prevention of cardiovascular disease (1.3) and hyperlipidemia (1.4). 1.1 Hypertension Amlodipine is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including amlodipine. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Amlodipine may be used alone or in combination with other antihypertensive agents. 1.2 Coronary Artery Disease (CAD) Chronic Stable Angina Amlodipine is indicated for the symptomatic treatment of chronic stable angina. Amlodipine may be used alone or in combination with other antianginal agents. Vasospastic Angina (Prinzmetal’s or Variant Angina) Amlodipine is indicated for the treatment of confirmed or suspected vasospastic angina. Amlodipine may be used as monotherapy or in combination with other antianginal agents. Angiographically Documented CAD In patients with recently documented CAD by angiography and without heart failure or an ejection fraction <40%, amlodipine is indicated to reduce the risk of hospitalization for angina and to reduce the risk of a coronary revascularization procedure. Atorvastatin Therapy with HMG CoA-reductase inhibitors (lipid-altering agents) should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease from hypercholesterolemia. Drug therapy is recommended as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In patients with coronary heart disease (CHD) or multiple risk factors for CHD, atorvastatin can be started simultaneously with diet restriction. 1.3 Prevention of Cardiovascular Disease (CVD) in Adults In adult patients without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as age, smoking, hypertension, low high-density lipoprotein cholesterol (HDL-C), or a family history of early coronary heart disease, atorvastatin is indicated to: Reduce the risk of myocardial infarction (MI) Reduce the risk of stroke Reduce the risk for revascularization procedures and angina In adult patients with type 2 diabetes, and without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as retinopathy, albuminuria, smoking, or hypertension, atorvastatin is indicated to: Reduce the risk of myocardial infarction Reduce the risk of stroke In adult patients with clinically evident coronary heart disease, atorvastatin is indicated to: Reduce the risk of non-fatal myocardial infarction Reduce the risk of fatal and non-fatal stroke Reduce the risk for revascularization procedures Reduce the risk of hospitalization for congestive heart failure (CHF) Reduce the risk of angina 1.4 Hyperlipidemia Atorvastatin is indicated: As an adjunct to diet to reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (apo B), and triglycerides (TG) levels and to increase HDL-C in adult patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia ( Fredrickson Types IIa and IIb) As an adjunct to diet for the treatment of adult patients with elevated serum TG levels ( Fredrickson Type IV); For the treatment of adult patients with primary dysbetalipoproteinemia ( Fredrickson Type III) who do not respond adequately to diet To reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable As an adjunct to diet to reduce total-C, LDL-C, and apo B levels in pediatric patients, 10 years to 17 years of age, with heterozygous familial hypercholesterolemia (HeFH) if after an adequate trial of diet therapy the following findings are present: a. LDL-C remains ≥ 190 mg/dL or b. LDL-C remains ≥ 160 mg/dL and: there is a positive family history of premature CVD or two or more other CVD risk factors are present in the pediatric patient 1.5 Limitations of Use Atorvastatin has not been studied in conditions where the major lipoprotein abnormality is elevation of chylomicrons ( Fredrickson Types I and V).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Amlodipine besylate and atorvastatin calcium Dosage of amlodipine besylate and atorvastatin calcium must be individualized on the basis of both effectiveness and tolerance for each individual component in the treatment of hypertension/angina and hyperlipidemia. Select doses of amlodipine and atorvastatin independently. Amlodipine besylate and atorvastatin calcium may be substituted for its individually titrated components. Patients may be given the equivalent dose of amlodipine besylate and atorvastatin calcium or a dose of amlodipine besylate and atorvastatin calcium with increased amounts of amlodipine, atorvastatin, or both for additional antianginal effects, blood pressure lowering, or lipid-lowering effect. Amlodipine besylate and atorvastatin calcium may be used to provide additional therapy for patients already on one of its components. Amlodipine besylate and atorvastatin calcium may be used to initiate treatment in patients with hyperlipidemia and either hypertension or angina. Amlodipine The usual initial antihypertensive oral dose of amlodipine is 5 mg once daily, and the maximum dose is 10 mg once daily. Pediatric (age > 6 years), small adult, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily and this dose may be used when adding amlodipine to other antihypertensive therapy. Adjust dosage according to blood pressure goals. In general, wait 7 to 14 days between titration steps. Titration may proceed more rapidly, however, if clinically warranted, provided the patient is assessed frequently. Angina: The recommended dose of amlodipine for chronic stable or vasospastic angina is 5 to 10 mg, with the lower dose suggested in the elderly and in patients with hepatic insufficiency. Most patients will require 10 mg for adequate effect. Coronary Artery Disease : The recommended dose range of amlodipine for patients with CAD is 5 to 10 mg once daily. In clinical studies, the majority of patients required 10 mg [see Clinical Studies ( 14.4 ) ]. Pediatrics: The effective antihypertensive oral dose of amlodipine in pediatric patients ages 6 to 17 years is 2.5 mg to 5 mg once daily. Doses in excess of 5 mg daily have not been studied in pediatric patients [see Clinical Pharmacology ( 12.3 ), Clinical Studies ( 14.1 ) ]. Atorvastatin (Hyperlipidemia) Hyperlipidemia and Mixed Dyslipidemia: The recommended starting dose of atorvastatin is 10 or 20 mg once daily. Patients who require a large reduction in LDL-C (more than 45%) may be started at 40 mg once daily. The dosage range of atorvastatin is 10 to 80 mg once daily. Atorvastatin can be administered as a single dose at any time of the day, with or without food. The starting dose and maintenance doses of atorvastatin should be individualized according to patient characteristics such as goal of therapy and response. After initiation and/or upon titration of atorvastatin, lipid levels should be analyzed within 2 to 4 weeks and dosage adjusted accordingly. Homozygous Familial Hypercholesterolemia: The dosage range of atorvastatin in patients with HoFH is 10 to 80 mg daily. Atorvastatin should be used as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) in these patients or if such treatments are unavailable. Concomitant Lipid-Lowering Therapy: Atorvastatin may be used with bile acid resins. Monitor for signs of myopathy in patients receiving the combination of HMG-CoA reductase inhibitors (statins) and fibrates [see Warnings and Precautions ( 5.1 ), Drug Interactions ( 7 ) ]. Patients with Renal Impairment: Renal disease does not affect the plasma concentrations nor LDL-C reduction of atorvastatin; thus, dosage adjustment in patients with renal dysfunction is not necessary [see Warnings and Precautions ( 5.1 ), Clinical Pharmacology ( 12.3 ) ]. Use with Cyclosporine, Clarithromycin, Itraconazole, Letermovir, or Certain Protease Inhibitors: In patients taking cyclosporine or the human immunodeficiency virus (HIV) protease inhibitor tipranavir plus ritonavir or the hepatitis C virus (HCV) protease inhibitor glecaprevir plus pibrentasvir, or letermovir when co-administered with cyclosporine, therapy with atorvastatin should be avoided. In patients with HIV taking lopinavir plus ritonavir, use the lowest dose necessary of atorvastatin. In patients taking clarithromycin, itraconazole, elbasvir plus grazoprevir, or in patients with HIV taking a combination of saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, or letermovir therapy with atorvastatin should be limited to 20 mg, and appropriate clinical assessment is recommended to ensure that the lowest dose necessary of atorvastatin is used. In patients taking the HIV protease inhibitor nelfinavir therapy with atorvastatin should be limited to 40 mg. [see Warnings and Precautions ( 5.1 ), Drug Interactions ( 7.3 )]. Heterozygous Familial Hypercholesterolemia in Pediatric Patients (10 Years to 17 Years of Age): The recommended starting dose of atorvastatin is 10 mg/day; the usual dose range is 10 to 20 mg orally once daily [see Clinical Studies ( 14.11 ) ]. Doses should be individualized according to the recommended goal of therapy [see Indications and Usage ( 1.4 ) and Clinical Pharmacology ( 12 ) ]. Adjustments should be made at intervals of 4 weeks or more. Usual starting dose (mg daily) Maximum dose (mg daily) Amlodipine 5 a 10 Atorvastatin 10 to 20 b 80 a Start small adults or children, fragile, or elderly patients, or patients with hepatic insufficiency on 2.5 mg once daily ( 2 ) b Start patients requiring large LDL-C reduction (>45%) at 40 mg once daily ( 2 )
