Drug Catalog - Product Detail
AMLODIPINE BENAZEPRIL CAPS 10/20MG 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 57237-0146-01 | RISING PHARMACEUTICALS | 100 | 10-20MG | CAPSULE |
PACKAGE FILES
Generic Name
AMLODIPINE AND BENAZEPRIL HYDROCHLORIDE
Substance Name
AMLODIPINE BESYLATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202239
Description
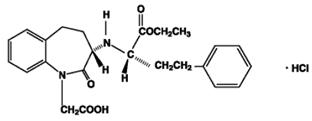
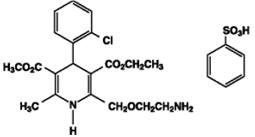
11 DESCRIPTION Amlodipine and benazepril hydrochloride capsules USP are a combination of amlodipine besylate and benazepril hydrochloride. Benazepril hydrochloride USP is a white to off-white, crystalline powder, soluble (greater than 100 mg/mL) in water, in ethanol, and in methanol. Benazepril hydrochloride’s chemical name is 3-[[1-(ethoxycarbonyl)-3-phenyl-(1S)-propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1 H -1-(3S)-benzazepine-1-acetic acid monohydrochloride; its structural formula is: Its molecular formula is C 24 H 28 N 2 O 5 •HCl, and its molecular weight is 460.96. Benazeprilat, the active metabolite of benazepril, is a nonsulfhydryl ACE inhibitor. Benazepril is converted to benazeprilat by hepatic cleavage of the ester group. Amlodipine besylate USP is a white or almost white powder, slightly soluble in water and sparingly soluble in ethanol. Its chemical name is (R,S)3-ethyl-5-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate benzenesulfonate; its structural formula is: Its molecular formula is C 20 H 25 ClN 2 O 5 •C 6 H 6 O 3 S, and its molecular weight is 567.1. Amlodipine besylate is the besylate salt of amlodipine, a dihydropyridine calcium channel blocker. Amlodipine and benazepril hydrochloride is available as capsules containing amlodipine besylate USP (3.5 mg, 6.9 mg or 13.9 mg, equivalent to 2.5 mg, 5 mg or 10 mg of amlodipine respectively), with 10 mg, 20 mg, or 40 mg of benazepril hydrochloride USP providing for the following available combinations: 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg, 5 mg/40 mg, 10 mg/20 mg, and 10 mg/40 mg. The inactive ingredients of the capsules are colloidal silicon dioxide, crospovidone, gelatin, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, and titanium dioxide. In addition, the hard gelatin capsule shells of 5 mg/10 mg contains iron oxide black, iron oxide red, and iron oxide yellow, 5 mg/20 mg contains iron oxide red, 5 mg/40 mg and 10 mg/40 mg contains FD&C Blue 1, FD&C Red 3, and 10 mg/20 mg contains D&C Red 28, FD&C Blue 1, FD&C Red 40, and FD&C Yellow 5. The capsules are printed with edible ink containing black iron oxide and shellac. Amlodipine Besylate Chemical Structure Benazepril Hydrochloride Chemical Structure
How Supplied
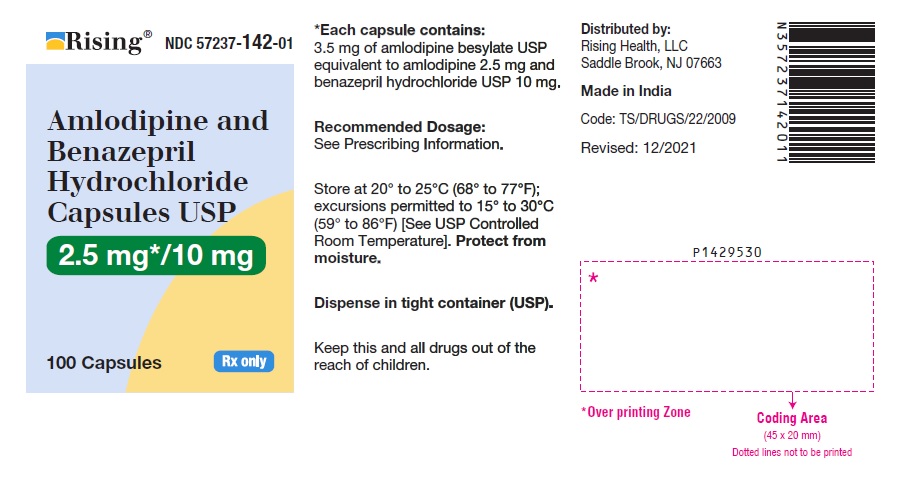
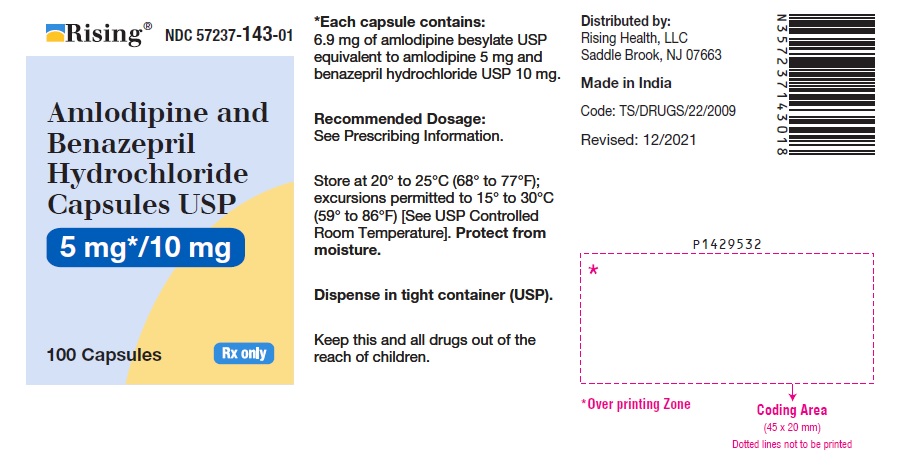
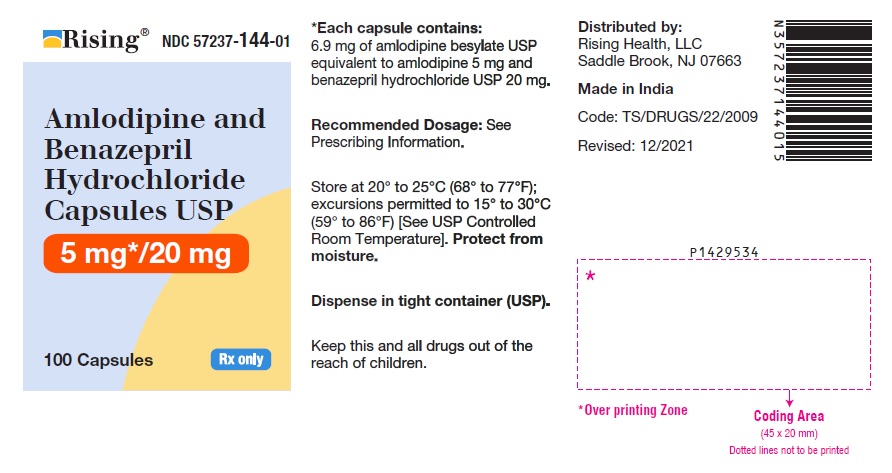
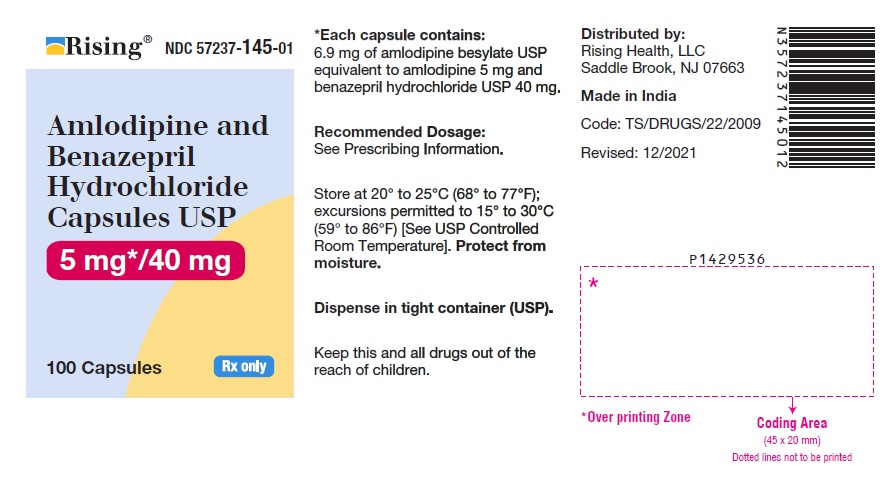
16 HOW SUPPLIED/STORAGE AND HANDLING Amlodipine and benazepril hydrochloride is available as capsules containing amlodipine besylate USP (3.5 mg, 6.9 mg or 13.9 mg, equivalent to 2.5 mg, 5 mg or 10 mg of amlodipine respectively), with 10 mg, 20 mg, or 40 mg of benazepril hydrochloride USP providing for the following available combinations: 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg, 5 mg/40 mg, 10 mg/20 mg, and 10 mg/40 mg. They are available as follows: Amlodipine and Benazepril Hydrochloride Capsules USP, 2.5 mg/10 mg are white to pale yellow colored powder filled in empty hard gelatin capsule shells, size “0” of white cap and white body imprinted with ‘I’ on white cap and ‘96’ on white body with black edible ink. Bottles of 100 NDC 57237-142-01 Bottles of 500 NDC 57237-142-05 Amlodipine and Benazepril Hydrochloride Capsules USP, 5 mg/10 mg are white to pale yellow colored powder filled in empty hard gelatin capsule shells, size “0” of light brown cap and light brown body imprinted with ‘I’ on light brown cap and ‘97’ on light brown body with black edible ink. Bottles of 100 NDC 57237-143-01 Bottles of 500 NDC 57237-143-05 Amlodipine and Benazepril Hydrochloride Capsules USP, 5 mg/20 mg are white to pale yellow colored powder filled in empty hard gelatin capsule shells, size “0” of pink cap and pink body imprinted with ‘I’ on pink cap and ‘98’ on pink body with black edible ink. Bottles of 100 NDC 57237-144-01 Bottles of 500 NDC 57237-144-05 Amlodipine and Benazepril Hydrochloride Capsules USP, 5 mg/40 mg are white to pale yellow colored powder filled in empty hard gelatin capsule shells, size “0” of light blue cap and light blue body imprinted with ‘J’ on light blue cap and ‘01’ on light blue body with black edible ink. Bottles of 100 NDC 57237-145-01 Bottles of 500 NDC 57237-145-05 Amlodipine and Benazepril Hydrochloride Capsules USP, 10 mg/20 mg are white to pale yellow colored powder filled in empty hard gelatin capsule shells, size “0” of purple cap and purple body imprinted with ‘J’ on purple cap and ‘02’ on purple body with black edible ink. Bottles of 100 NDC 57237-146-01 Bottles of 500 NDC 57237-146-05 Amlodipine and Benazepril Hydrochloride Capsules USP, 10 mg/40 mg are white to pale yellow colored powder filled in empty hard gelatin capsule shells, size “0” of dark blue cap and dark blue body imprinted with ‘J’ on dark blue cap and ‘03’ on dark blue body with black edible ink. Bottles of 100 NDC 57237-147-01 Bottles of 500 NDC 57237-147-05 Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from moisture. Dispense in tight container (USP).
Indications & Usage
1 INDICATIONS AND USAGE Amlodipine and benazepril hydrochloride capsules are a combination capsule of amlodipine, a dihydropyridine calcium channel blocker (DHP CCB) and benazepril, an angiotensin-converting enzyme (ACE) inhibitor. Amlodipine and benazepril hydrochloride capsules are indicated for the treatment of hypertension in patients not adequately controlled on monotherapy with either agent. (1) 1.1 Hypertension Amlodipine and benazepril hydrochloride capsules are indicated for the treatment of hypertension in patients not adequately controlled on monotherapy with either agent.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Usual starting dose is 2.5 mg/10 mg. ( 2.1 ) May be used as add-on therapy for patients not adequately controlled with either a dihydropyridine calcium channel blocker or an ACE inhibitor ( 2.2 ) Patients who experience edema with amlodipine may be switched to amlodipine and benazepril hydrochloride capsules containing a lower dose of amlodipine. ( 2.1 ) 2.1 General Considerations The recommended initial dose is amlodipine 2.5 mg and benazepril 10 mg orally once-daily. Begin therapy with amlodipine and benazepril hydrochloride capsules only after a patient has either (a) failed to achieve the desired antihypertensive effect with amlodipine or benazepril monotherapy, or (b) demonstrated inability to achieve adequate antihypertensive effect with amlodipine therapy without developing edema. The antihypertensive effect of amlodipine and benazepril hydrochloride capsules is largely attained within 2 weeks. If blood pressure remains uncontrolled, the dose may be titrated up to amlodipine 10 mg and benazepril 40 mg once-daily. The dosing should be individualized and adjusted according to the patient’s clinical response. In clinical trials of amlodipine and benazepril combination therapy using amlodipine doses of 2.5 to 10 mg and benazepril doses of 10 to 40 mg, the antihypertensive effects increased with increasing dose of amlodipine in all patient groups, and the effects increased with increasing dose of benazepril in nonblack groups. 2.2 Replacement Therapy Amlodipine and benazepril hydrochloride capsules may be substituted for the titrated components.
