Drug Catalog - Product Detail
AMIODARONE HCL TAB 100 MG 30 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 51862-0240-30 | MAYNE PHARMA | 30 | 100MG | TABLET |
PACKAGE FILES


Generic Name
AMIODARONE HYDROCHLORIDE
Substance Name
AMIODARONE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075389
Description
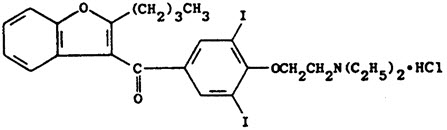
11 DESCRIPTION Amiodarone hydrochloride tablets, USP is an antiarrhythmic drug, available for oral administration as white tablets containing 100 mg of amiodarone hydrochloride, white, scored tablets containing 200 mg of amiodarone hydrochloride, and yellow, scored tablets containing 400 mg of amiodarone hydrochloride. The inactive ingredients present are lactose, starch, microcrystalline cellulose, sodium starch glycolate, povidone, colloidal silicon dioxide, magnesium stearate, and D&C Yellow #10. Amiodarone is a benzofuran derivative: 2-butyl-3-benzofuranyl 4-[2-(diethylamino)-ethoxy]- 3,5-diiodophenyl ketone hydrochloride. The structural formula is as follows: Amiodarone HCl is a white to cream-colored crystalline powder. It is slightly soluble in water, soluble in alcohol, and freely soluble in chloroform. It contains 37.3% iodine by weight. Meets USP Dissolution Test 4. FDA approved dissolution test specifications differ from USP. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Amiodarone hydrochloride tablets, USP are available as follows: 100 mg, white, round, flat-faced, beveled-edge tablet. Debossed with "m" on the upper portion and "154" on the lower half on one side of the tablet, and plain on the other. Bottles of 30 tablets NDC 51862-240-30 200 mg, white, round, flat-faced, beveled-edge, scored tablet. Debossed with "m" on the upper portion and "155" on the lower half on one side of the tablet with a score mark between and plain on the other. Bottles of 60 tablets NDC 51862-241-60 Bottles of 90 tablets NDC 51862-241-90 Bottles of 500 tablets NDC 51862-241-05 400 mg, Oval-shaped, convex, yellow, scored tablet. Debossed with "m" on the left and "156" on the right with a score mark between on one side, and plain on the other. Bottles of 30 tablets NDC 51862-242-30 Keep tightly closed. Store at Controlled Room Temperature, 20° to 25°C (68° to 77°F). Protect from light. Dispense in a light-resistant, tight container.
Indications & Usage
1 INDICATIONS AND USAGE Amiodarone hydrochloride tablets are indicated for the treatment of documented, life-threatening recurrent ventricular fibrillation and life-threatening recurrent hemodynamically unstable tachycardia in adults who have not responded to adequate doses of other available antiarrhythmics or when alternative agents cannot be tolerated. Amiodarone hydrochloride tablets is an antiarrhythmic indicated for: Recurrent ventricular fibrillation. ( 1 ) Recurrent hemodynamically unstable ventricular tachycardia. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Dosage must be individualized based on severity of arrhythmia and response. Use the lowest effective dose. Obtain baseline chest x-ray, pulmonary function tests, thyroid function tests, and liver aminotransferases. Correct hypokalemia, hypomagnesemia, and hypocalcemia before initiating treatment. Initiate treatment with a loading doses of 800 to 1600 mg/day until initial therapeutic response occurs (usually 1 to 3 weeks). Once adequate arrhythmia control is achieved, or if side effects become prominent, reduce amiodarone hydrochloride tablets dose to 600 to 800 mg/day for one month and then to the maintenance dose, usually 400 mg/day. ( 2 ) Recommended Dosage: Initiate treatment with a loading doses of 800 to 1600 mg/day until initial therapeutic response occurs (usually 1 to 3 weeks). Once adequate arrhythmia control is achieved, or if side effects become prominent, reduce amiodarone hydrochloride tablets dose to 600 to 800 mg/day for one month and then to the maintenance dose, usually 400 mg/day. Administration: Administer amiodarone hydrochloride tablets consistently with regard to meals [see Clinical Pharmacology (12.3) ] . Administration of amiodarone hydrochloride tablets in divided doses with meals is suggested for total daily doses of 1000 mg or higher, or when gastrointestinal intolerance occurs.
