Drug Catalog - Product Detail
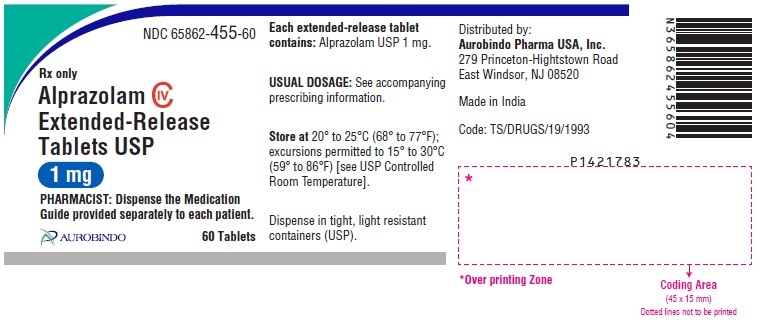
ALPRAZOLAM TAB ER 24HR 1MG 60CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0455-60 | AUROBINDO PHARMA | 60 | 1MG | TABLET |
PACKAGE FILES
Generic Name
ALPRAZOLAM
Substance Name
ALPRAZOLAM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090871
Description
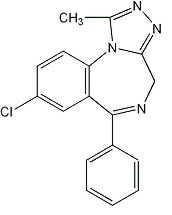
11 DESCRIPTION Alprazolam extended-release tablets USP contain alprazolam which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds. The chemical name of alprazolam is 8-chloro-1-methyl-6-phenyl-4 H - s -triazolo [4,3-α] [1,4] benzodiazepine. The molecular formula is C 17 H 13 ClN 4 which corresponds to a molecular weight of 308.76. The structural formula is represented below: Alprazolam USP is a white to off-white, crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH. Each alprazolam extended-release tablet USP, for oral administration, contains 0.5 mg, 1 mg, 2 mg, or 3 mg of alprazolam USP. The inactive ingredients are colloidal silicon dioxide, hypromellose, lactose monohydrate, and magnesium stearate. In addition, the 1 mg and 3 mg tablets contain D&C Yellow No. 10 aluminum lake and the 2 mg and 3 mg tablets contain FD&C Blue No. 2 lake. Meets USP Dissolution Test 4. Chemical Structure
How Supplied
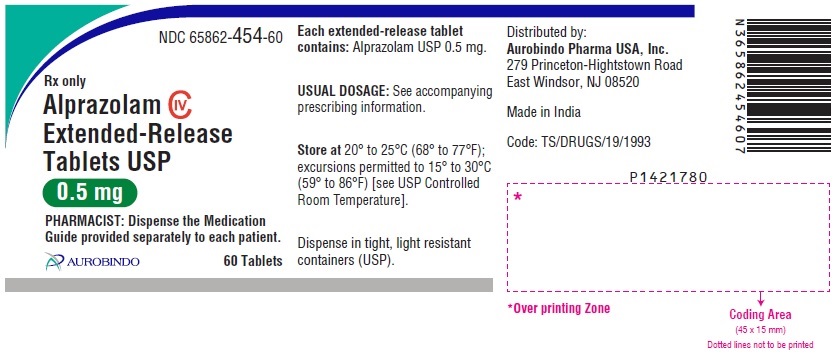
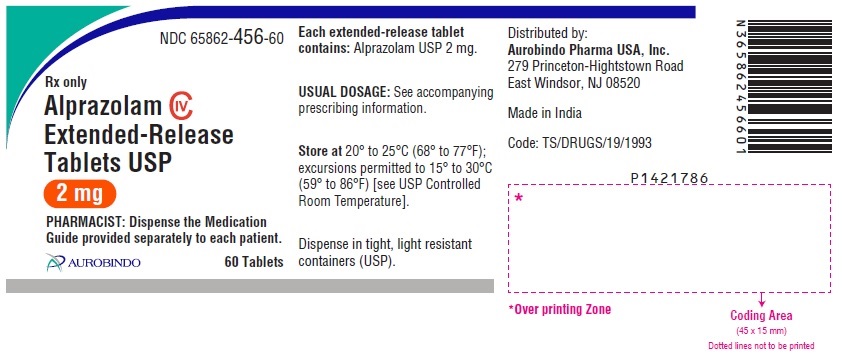
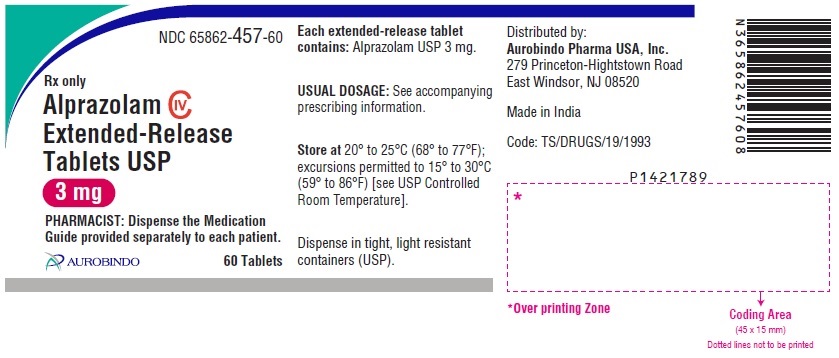
16 HOW SUPPLIED/STORAGE AND HANDLING Alprazolam Extended-Release Tablets USP are supplied in the following strengths and package configurations: Alprazolam Extended-Release Tablets USP, 0.5 mg are white to off-white, round, biconvex tablets with beveled edge debossed with ‘X’ on one side and ‘70’ on the other side. Bottles of 60 NDC 65862-454-60 Bottles of 1,000 NDC 65862-454-99 Bottles of 7,000 NDC 65862-454-71 Alprazolam Extended-Release Tablets USP, 1 mg are yellow colored, round, biconvex tablets with beveled edge debossed with ‘X’ on one side and ‘73’ on the other side. The tablets may be mottled. Bottles of 60 NDC 65862-455-60 Bottles of 1,000 NDC 65862-455-99 Bottles of 7,000 NDC 65862-455-71 Alprazolam Extended-Release Tablets USP, 2 mg are blue colored, round, biconvex tablets with beveled edge debossed with ‘X’ on one side and ‘74’ on the other side. The tablets may be mottled. Bottles of 60 NDC 65862-456-60 Bottles of 1,000 NDC 65862-456-99 Bottles of 7,000 NDC 65862-456-71 Alprazolam Extended-Release Tablets USP, 3 mg are green colored, round, biconvex tablets with beveled edge debossed with ‘X’ on one side and ‘75’ on the other side. The tablets may be mottled. Bottles of 60 NDC 65862-457-60 Bottles of 1,000 NDC 65862-457-99 Bottles of 7,000 NDC 65862-457-71 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Alprazolam extended-release tablets are indicated for the treatment of panic disorder with or without agoraphobia, in adults. Alprazolam extended-release tablets are a benzodiazepine indicated for the treatment of panic disorder with or without agoraphobia, in adults. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Recommended starting oral dosage is 0.5 mg to 1 mg once daily (preferably in the morning). Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg daily. ( 2.1 ) Recommended total daily dosage is 3 mg to 6 mg daily. ( 2.1 ) Swallow tablets whole; do not divide, crush, or chew. ( 2.1 ) When tapering, decrease dosage by no more than 0.5 mg every 3 days. Some patients may require an even slower dosage reduction. ( 2.2 , 5.2 ) See the Full Prescribing Information for the recommended dosage in geriatric patients, patients with hepatic impairment, and with use with ritonavir. ( 2.3 , 2.4 , 2.5 ) 2.1 Recommended Dosage Administer alprazolam extended-release tablets orally once daily, preferably in the morning. Swallow tablets whole; do not divide, crush, or chew. The recommended starting oral dosage for alprazolam extended-release tablets are 0.5 mg to 1 mg once daily. Depending on the response, the dosage may be adjusted at intervals of every 3 to 4 days in increments of no more than 1 mg daily. The recommended dosage range is 3 mg to 6 mg once daily. Controlled trials of alprazolam extended-release tablets for the treatment of panic disorder included dosages in the range of 1 mg to 10 mg per day. Most patients showed a response in the dosage range of 3 mg to 6 mg per day. Occasional patients required as much as 10 mg per day. The longer-term efficacy of alprazolam extended-release tablets has not been systematically evaluated. If alprazolam extended-release tablets are used for periods longer than 8 weeks, the healthcare provider should periodically reassess the usefulness of the drug for the individual patient. After a period of extended freedom from panic attacks, a carefully supervised tapered discontinuation may be attempted, but there is evidence that this may often be difficult to accomplish without recurrence of symptoms and/or the manifestation of withdrawal phenomena [see Dosage and Administration (2.2) , Warnings and Precautions (5.2) ]. 2.2 Discontinuation or Dosage Reduction of Alprazolam Extended-Release Tablets To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam extended-release tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly [see Warnings and Precautions (5.3) , Drug Abuse and Dependence (9.3) ]. Reduce the dosage by no more than 0.5 mg every three days. Some patients may benefit from an even more gradual discontinuation. Some patients may prove resistant to all discontinuation regimens. In a controlled postmarketing discontinuation study of panic disorder patients which compared the recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome. 2.3 Dosage Recommendations in Geriatric Patients In geriatric patients, the recommended starting dosage of alprazolam extended-release tablets is 0.5 mg once daily. This may be gradually increased if needed and tolerated. Geriatric patients may be sensitive to the effects of benzodiazepines [see Use in Specific Populations (8.5) , Clinical Pharmacology (12.3) ]. 2.4 Dosage Recommendations in Patients with Hepatic Impairment In patients with hepatic impairment, the recommended starting dosage of alprazolam extended-release tablets is 0.5 mg once daily. This may be gradually increased if needed and tolerated [see Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ] . 2.5 Dosage Modifications for Drug Interactions Alprazolam extended-release tablets should be reduced to half of the recommended dosage when a patient is started on ritonavir and alprazolam extended-release tablets together, or when ritonavir is added to a patient treated with alprazolam extended-release tablets. Increase alprazolam extended-release tablets dosage to the target dose after 10 to 14 days of dosing ritonavir and alprazolam extended-release tablets together. It is not necessary to reduce alprazolam extended-release tablets dosage in patients who have been taking ritonavir for more than 10 to 14 days. Alprazolam extended-release tablets are contraindicated with concomitant use of all strong CYP3A inhibitors, except ritonavir [see Contraindications (4) , Warnings and Precautions (5.5) , Drug Interactions (7.1) ]. 2.6 Switching Patients from Alprazolam Tablets to Alprazolam Extended-Release Tablets Patients who are currently being treated with divided doses of alprazolam may be switched to alprazolam extended-release tablets at the same total daily dose taken once daily. If the clinical response after switching is inadequate, titrate the dosage as outlined above.
