Drug Catalog - Product Detail
ALOGLIPTIN TABS 12.5MG 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0103-65 | PADAGIS | 30 | 12.5MG | TABLET |
PACKAGE FILES


Generic Name
ALOGLIPTIN
Substance Name
ALOGLIPTIN BENZOATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA022271
Description
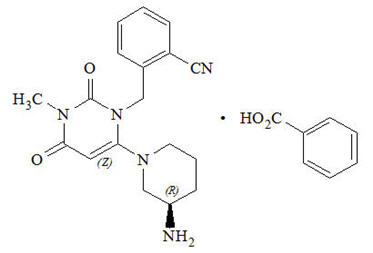
11 DESCRIPTION Alogliptin tablets contain the active ingredient alogliptin, which is a selective, orally bioavailable inhibitor of the enzymatic activity of dipeptidyl peptidase-4 (DPP-4). Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-({6-[(3 R )-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2 H )-yl}methyl)benzonitrile monobenzoate. It has a molecular formula of C 18 H 21 N 5 O 2 ∙C 7 H 6 O 2 and a molecular weight of 461.51 daltons. The structural formula is: Alogliptin benzoate is a white to off-white crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol and very slightly soluble in octanol and isopropyl acetate. Each alogliptin tablet contains 34 mg, 17 mg or 8.5 mg alogliptin benzoate, which is equivalent to 25 mg, 12.5 mg or 6.25 mg, respectively, of alogliptin and the following inactive ingredients: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium and magnesium stearate. In addition, the film coating contains the following inactive ingredients: hypromellose, titanium dioxide, ferric oxide (red or yellow) and polyethylene glycol, and is marked with printing ink (Gray F1). Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Alogliptin tablets are available as film-coated tablets containing 25 mg, 12.5 mg or 6.25 mg of alogliptin as follows: 25 mg tablet: light red, oval, biconvex, film-coated, with "TAK ALG-25" printed on one side, available in: NDC 45802-150-65 Bottles of 30 tablets 12.5 mg tablet: yellow, oval, biconvex, film-coated, with "TAK ALG-12.5" printed on one side, available in: NDC 45802-103-65 Bottles of 30 tablets 6.25 mg tablet: light pink, oval, biconvex, film-coated, with "TAK ALG-6.25" printed on one side, available in: NDC 45802-087-65 Bottles of 30 tablets Storage Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
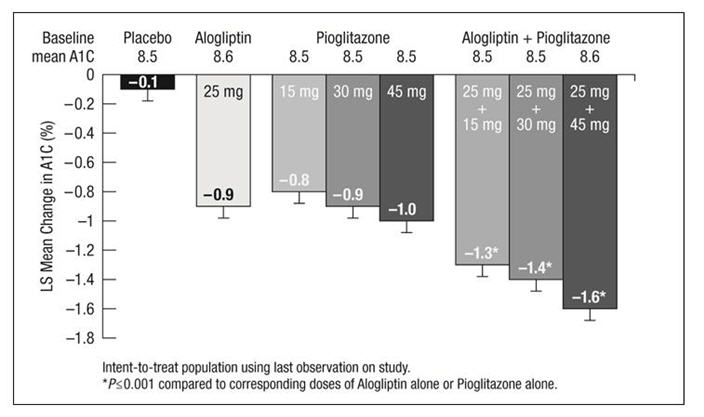
1 INDICATIONS AND USAGE Alogliptin tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14) ]. Alogliptin tablets are a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. ( 1 ) Limitations of Use: Should not be used in patients with type 1 diabetes. ( 1 ) Limitations of Use Alogliptin tablets should not be used in patients with type 1 diabetes mellitus.
Dosage and Administration
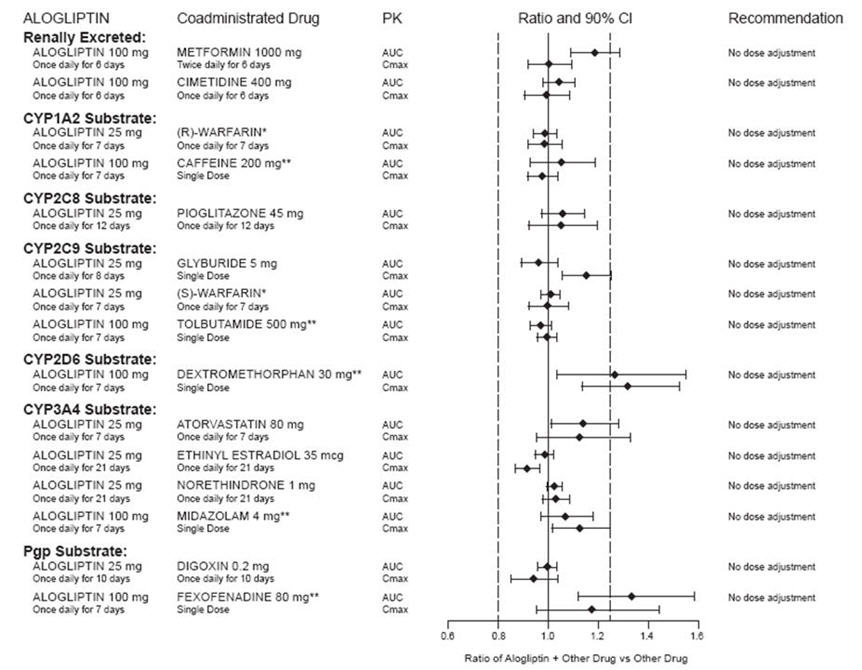
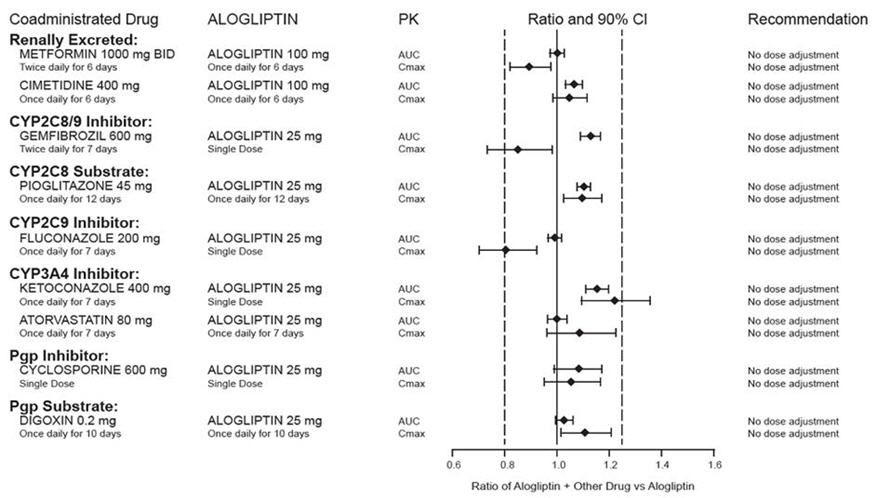
2 DOSAGE AND ADMINISTRATION The recommended dose in patients with normal renal function or mild renal impairment is 25 mg once daily. ( 2.1 ) Can be taken with or without food. ( 2.1 ) Adjust dose if moderate or severe renal impairment or end-stage renal disease (ESRD). ( 2.2 ) Degree of Renal Impairment Creatinine Clearance (mL/min) Recommended Dosing Moderate ≥30 to <60 12.5 mg once daily Severe/ESRD <30 6.25 mg once daily 2.1 Recommended Dosing The recommended dose of alogliptin tablets is 25 mg once daily. Alogliptin tablets may be taken with or without food. Do not split tablets. 2.2 Patients with Renal Impairment No dose adjustment of alogliptin tablets is necessary for patients with mild renal impairment (creatinine clearance [CrCl] ≥60 mL/min). The dose of alogliptin tablets is 12.5 mg once daily for patients with moderate renal impairment (CrCl ≥30 to <60 mL/min). The dose of alogliptin tablets is 6.25 mg once daily for patients with severe renal impairment (CrCl ≥15 to <30 mL/min) or with end-stage renal disease (ESRD) (CrCl <15 mL/min or requiring hemodialysis). Alogliptin tablets may be administered without regard to the timing of dialysis. Alogliptin tablets have not been studied in patients undergoing peritoneal dialysis [see Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ]. Because there is a need for dose adjustment based upon renal function, assessment of renal function is recommended prior to initiation of alogliptin tablets therapy and periodically thereafter.
