Drug Catalog - Product Detail
ALOGLIPTIN AND PIOGLITAZONE TB 12.5MG/30MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0260-65 | PADAGIS | 30 | 12.5-30MG | NA |
PACKAGE FILES





Generic Name
ALOGLIPTIN BENZOATE AND PIOGLITAZONE HYDROCHLORIDE
Substance Name
ALOGLIPTIN BENZOATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA022426
Description
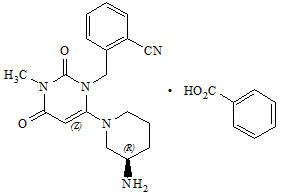
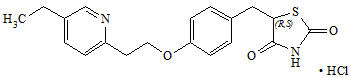
11 DESCRIPTION Alogliptin and pioglitazone tablets contain two oral antihyperglycemic drugs used in the management of type 2 diabetes mellitus: alogliptin and pioglitazone. Alogliptin Alogliptin is a selective, orally bioavailable inhibitor of the enzymatic activity of dipeptidyl peptidase-4 (DPP-4). Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-({6-[(3 R )-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2 H )-yl}methyl)benzonitrile monobenzoate. It has a molecular formula of C 18 H 21 N 5 O 2 ∙C 7 H 6 O 2 and a molecular weight of 461.51 daltons. The structural formula is: Alogliptin benzoate is a white to off-white crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol and very slightly soluble in octanol and isopropyl acetate. Chemical Structure Pioglitazone Chemically, pioglitazone is prepared as hydrochloride (HCl) salt, which is identified as (±)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione monohydrochloride. It has a molecular formula of C 19 H 20 N 2 O 3 S∙HCl and a molecular weight of 392.90 daltons. The structural formula is: Pioglitazone HCl is an odorless white crystalline powder that contains one asymmetric carbon in the thiazolidinedione moiety. The synthetic compound is a racemate and the two enantiomers of pioglitazone interconvert in vivo . It is soluble in N,N dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water and insoluble in ether. Alogliptin and pioglitazone tablets are available as a fixed-dose combination tablet for oral administration containing 34 mg alogliptin benzoate equivalent to 25 mg alogliptin and any of the following strengths of pioglitazone HCl: 16.53 mg pioglitazone HCl equivalent to 15 mg pioglitazone (25 mg/15 mg) 33.06 mg pioglitazone HCl equivalent to 30 mg pioglitazone (25 mg/30 mg) 49.59 mg pioglitazone HCl equivalent to 45 mg pioglitazone (25 mg/45 mg) Alogliptin and pioglitazone tablets are also available as a fixed-dose combination tablet for oral administration containing 17 mg alogliptin benzoate equivalent to 12.5 mg alogliptin for the following strength of pioglitazone HCl: 33.06 mg pioglitazone HCl equivalent to 30 mg pioglitazone (12.5 mg/30 mg) Alogliptin and pioglitazone tablets contain the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, mannitol, and microcrystalline cellulose; the tablets are film-coated with ferric oxide (yellow and/or red), hypromellose, polyethylene glycol, talc, titanium dioxide, and are marked with printing ink (Red A1 or Gray F1). Chemical Structure
How Supplied
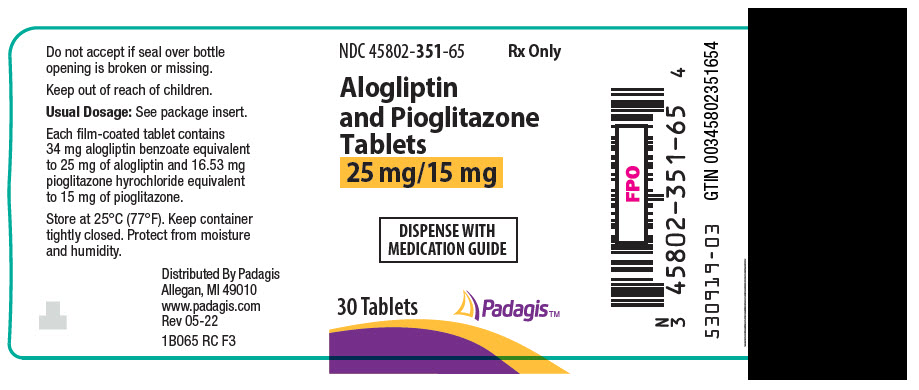
16 HOW SUPPLIED/STORAGE AND HANDLING Alogliptin and pioglitazone tablets are available in the following strengths and packages: 25 mg/15 mg tablet: yellow, round, biconvex and film-coated with both “A/P” and “25/15” printed on one side, available in: NDC 45802-351-65 Bottles of 30 tablets 25 mg/30 mg tablet: peach, round, biconvex and film-coated with both “A/P” and “25/30” printed on one side, available in: NDC 45802-402-65 Bottles of 30 tablets 25 mg/45 mg tablet: red, round, biconvex, film-coated and with both “A/P” and “25/45” printed on one side, available in: NDC 45802-499-65 Bottles of 30 tablets 12.5 mg/30 mg tablet: pale peach, round, biconvex and film-coated with both “A/P” and “12.5/30” printed on one side, available in: NDC 45802-260-65 Bottles of 30 tablets Storage Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed and protect from moisture and humidity.
Indications & Usage
1 INDICATIONS AND USAGE Alogliptin and pioglitazone tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus . Alogliptin and pioglitazone tablets are a combination of alogliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone, a thiazolidinedione agonist of peroxisome proliferator receptor gamma, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. ( 1 ) Limitations of Use: Alogliptin and pioglitazone tablets are not recommended for use in patients with type 1 diabetes mellitus. ( 1 ) Limitations of Use Alogliptin and pioglitazone tablets are not recommended for use in patients with type 1 diabetes mellitus.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Obtain liver tests prior to initiation. Alogliptin and pioglitazone tablets may be taken with or without food. ( 2.1 ) Individualize the starting dose of alogliptin and pioglitazone tablets based on the patient’s current regimen and concurrent medical condition but do not exceed a daily dose of alogliptin 25 mg and pioglitazone 45 mg. ( 2.2 ) The recommended starting dosage in patients with NYHA Class I or II congestive heart failure is 25 mg of alogliptin and 15 mg of pioglitazone ( 2.4 ) Prior to initiation, assess renal function with creatinine clearance (CrCl) ( 2.3 ) Mild renal impairment (creatinine clearance [CrCl] ≥60 mL/min): same as the recommended dosage in patients with normal renal function. Moderate renal impairment (CrCl ≥30 to <60 mL/min): 12.5 mg of alogliptin and 30 mg of pioglitazone once daily. Severe renal impairment (CrCl ≥15 to <30 mL/min) or ESRD (CrCl <15 mL/min or requiring hemodialysis): not recommended. 2.1 Important Dosage and Administration Information Obtain liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) prior to initiating alogliptin and pioglitazone tablets [see Warnings and Precautions (5.4) ] . Take alogliptin and pioglitazone tablets orally once daily. Do not split tablets. Alogliptin and pioglitazone tablets may be taken with or without food [see Clinical Pharmacology (12.3) ] . If a dose is missed, do not double the next dose. 2.2 Recommended Dosage and Administration Recommended Starting Dosage Based on Current Regimen Individualize the starting dosage of alogliptin and pioglitazone tablets based on the patient's current regimen and the available strengths of alogliptin and pioglitazone tablets (see Table 1 ). Table 1: Recommended Starting Dosage Based on the Patient’s Current Regimen Current Regimen Starting Dosage of Alogliptin and Pioglitazone Tablets For dosage recommendations for patients with renal impairment and/or congestive heart failure, [see Dosage and Administration (2.3 , 2.4) ] . Not treated with either alogliptin or pioglitazone 25 mg/15 mg or 25 mg/30 mg Alogliptin 25 mg/15 mg or 25 mg/30 mg Pioglitazone 25 mg/15 mg, 25 mg/30 mg, or 25 mg/45 mg Alogliptin and pioglitazone Select a dosage that is as close as possible to the current dosage of alogliptin and pioglitazone Dosage Titration for Additional Glycemic Control Titrate the alogliptin and pioglitazone tablets dosage gradually, as needed, after assessing therapeutic response and tolerability, up to a maximum dosage of 25 mg of alogliptin and 45 mg of pioglitazone once daily. 2.3 Recommended Dosage for Patients with Renal Impairment Assess renal function prior to initiation of alogliptin and pioglitazone tablets and periodically thereafter [see Use in Specific Populations (8.6) ] . The recommended dosage of alogliptin and pioglitazone tablets in patients with mild renal impairment (creatinine clearance [CrCl] ≥60 mL/min) is the same as the recommended dosage in patients with normal renal function [see Dosage and Administration (2.1) ] . The recommended dosage of alogliptin and pioglitazone tablets for patients with moderate renal impairment (CrCl ≥30 to <60 mL/min) is 12.5 mg of alogliptin and 30 mg of pioglitazone once daily. Alogliptin and pioglitazone tablets are not recommended for patients with severe renal impairment (CrCl ≥15 to <30 mL/min) or ESRD (CrCl <15 mL/min or requiring hemodialysis) because these patients require a lower dosage of alogliptin than what is available in the fixed dose combination product, alogliptin and pioglitazone tablets [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3) ] . 2.4 Recommendations for Congestive Heart Failure Starting Dosage in Patients with NYHA Class I or II Congestive Heart Failure For patients with preexisting NYHA Class I or II congestive heart failure, the recommended starting dosage of alogliptin and pioglitazone tablets is 25 mg of alogliptin and 15 mg of pioglitazone [see Boxed Warning and Warnings and Precautions (5.1) ] . Monitoring for Fluid Retention and Dosage Modifications for Congestive Heart Failure After initiation of alogliptin and pioglitazone tablets or with dosage increase, monitor patients carefully for adverse reactions related to fluid retention as has been seen with pioglitazone (e.g., weight gain, edema and signs and symptoms of congestive heart failure). If congestive heart failure develops while taking alogliptin and pioglitazone tablets, consider discontinuation of alogliptin and pioglitazone tablets or dosage reduction of pioglitazone in alogliptin and pioglitazone tablets [see Boxed Warning and Warnings and Precautions (5.1) ] . 2.5 Coadministration with Strong CYP2C8 Inhibitors The maximum recommended dosage of alogliptin and pioglitazone tablets is 25 mg of alogliptin and 15 mg of pioglitazone once daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ] .
