Drug Catalog - Product Detail
ALOGLIPTIN AND METFORMIN HCL TB 12.5MG/500MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0169-72 | PADAGIS | 60 | 12.5-500MG | TABLET |
PACKAGE FILES


Generic Name
ALOGLIPTIN AND METFORMIN HYDROCHLORIDE
Substance Name
ALOGLIPTIN BENZOATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA203414
Description
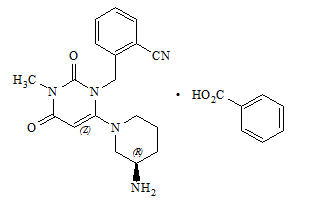
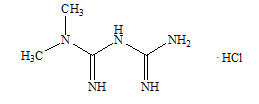
11 DESCRIPTION Alogliptin and metformin HCl tablets contain two oral antihyperglycemic drugs used in the management of type 2 diabetes mellitus: alogliptin and metformin HCl. Alogliptin Alogliptin is a selective, orally bioavailable inhibitor of the enzymatic activity of DPP-4. Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-({6-[(3 R )-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2 H )-yl}methyl)benzonitrile monobenzoate. It has a molecular formula of C 18 H 21 N 5 O 2 ∙C 7 H 6 O 2 and a molecular weight of 461.51 daltons; the structural formula is: Alogliptin benzoate is a white to off-white crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol and very slightly soluble in octanol and isopropyl acetate. Chemical Structure Metformin HCl Metformin HCl ( N,N -dimethylimidodicarbonimidic diamide hydrochloride) is not chemically or pharmacologically related to any other classes of oral antihyperglycemic agents. Metformin HCl is a white to off-white crystalline compound with a molecular formula of C 4 H 11 N 5 ∙HCl and a molecular weight of 165.63. Metformin HCl is freely soluble in water and is practically insoluble in acetone, ether and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin HCl is 6.68. The structural formula is as shown: Alogliptin and metformin HCl tablets are available as a tablet for oral administration containing 17 mg alogliptin benzoate equivalent to 12.5 mg alogliptin and: 500 mg metformin HCl which is equivalent to 389.93 mg metformin base (12.5 mg/500 mg) or 1000 mg metformin HCl which is equivalent to 779.86 mg metformin base (12.5 mg/1000 mg). Alogliptin and metformin HCl tablets contain the following inactive ingredients: crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, and povidone; the tablets are film-coated with ferric oxide yellow, hypromellose 2910, talc, and titanium dioxide. Chemical Structure
How Supplied
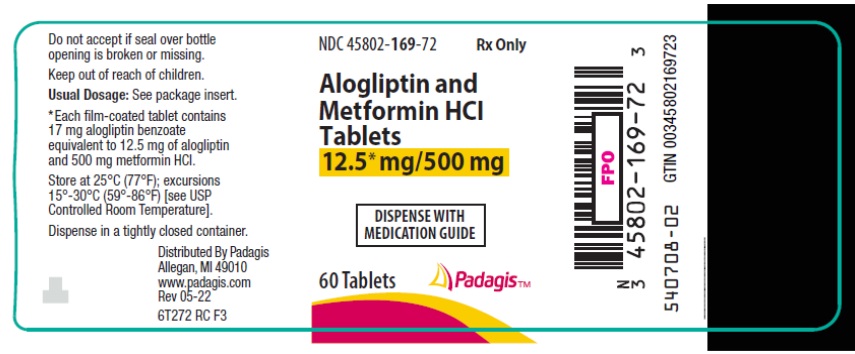
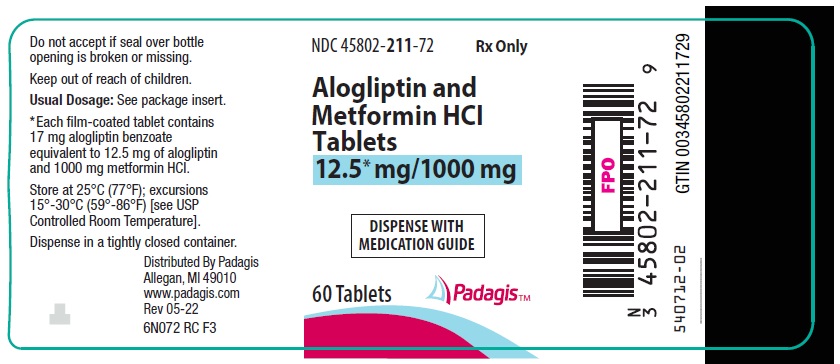
16 HOW SUPPLIED/STORAGE AND HANDLING Alogliptin and metformin HCl tablets are available in the following strengths and packages: 12.5 mg/500 mg tablet: pale yellow, oblong, film-coated tablets with "12.5/500" debossed on one side and "322M" debossed on the other side, available in: NDC 45802-169-72 Bottles of 60 tablets 12.5 mg/1000 mg tablet: pale yellow, oblong, film-coated tablets with "12.5/1000" debossed on one side and "322M" debossed on the other side, available in: NDC 45802-211-72 Bottles of 60 tablets Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed.
Indications & Usage
1 INDICATIONS AND USAGE Alogliptin and metformin HCl tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Alogliptin and metformin HCl tablets are a combination of alogliptin, a dipeptidyl-peptidase-4 (DPP-4) inhibitor and metformin hydrochloride (HCl), a biguanide, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. ( 1 ) Limitations of Use: Should not be used in patients with type 1 diabetes mellitus. ( 1 ) Limitations of Use Alogliptin and metformin HCl tablets should not recommended for use in patients with type 1 diabetes mellitus.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Individualize the starting dosage based on the patient's current regimen. ( 2.1 ) Given orally twice daily with food. ( 2.1 ) Adjust the dosage based on effectiveness and tolerability while not exceeding the maximum recommended daily dosage of 25 mg alogliptin and 2000 mg metformin HCl. ( 2.1 ) Prior to initiation, assess renal function with estimated glomerular filtration rate (eGFR). ( 2.2 ) Do not use in patients with eGFR below 60 mL/min/1.73 m 2 . Alogliptin and metformin HCl tablets may need to be discontinued at time of, or prior to, iodinated contrast imaging procedures. ( 2.3 ) 2.1 Recommended Dosage Individualize the starting dosage of alogliptin and metformin HCl tablets based on the patient’s current regimen. Alogliptin and metformin HCl tablets should be taken orally twice daily with food with gradual dose escalation to reduce the gastrointestinal (GI) side effects due to metformin. Do not split tablets. Adjust the dosage based on effectiveness and tolerability while not exceeding the maximum recommended daily dose of 25 mg alogliptin and 2000 mg metformin hydrochloride (HCl). 2.2 Recommendations for Use in Renal Impairment Assess renal function prior to initiation of alogliptin and metformin HCl tablets and periodically thereafter. Alogliptin and metformin HCl tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m 2 [see Contraindications (4) , Warnings and Precautions (5.1) ] . Alogliptin and metformin HCl tablets are not recommended in patients with an eGFR between 30 and 59 mL/min/1.73 m 2 because these patients require a lower daily dosage of alogliptin than what is available in the fixed combination alogliptin and metformin HCl tablets product. Alogliptin and metformin HCl tablets require no dose adjustment in patients with an eGFR of 60 mL/min/1.73 m 2 or greater. 2.3 Discontinuation for Iodinated Contrast Imaging Procedures Discontinue alogliptin and metformin HCl tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m 2 ; in patients with a history of liver disease, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart alogliptin and metformin HCl tablets if renal function is stable [see Warnings and Precautions (5.1) ] .
