Drug Catalog - Product Detail
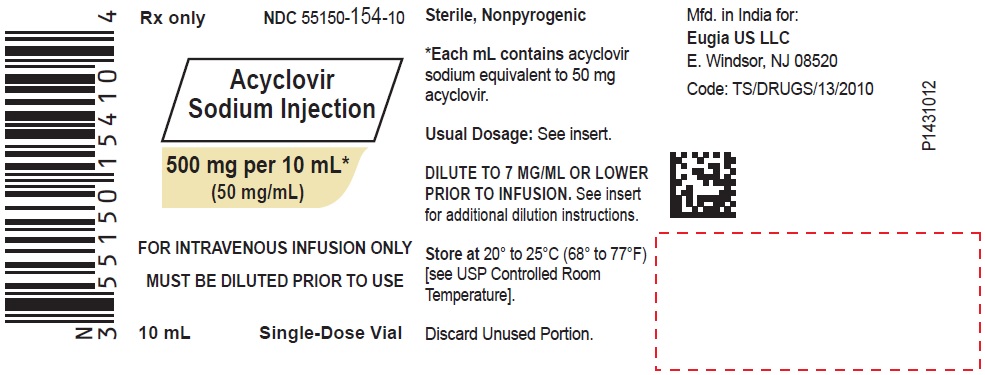
ACYCLOVIR SODIUM INJECTION INJECT. 500MG/10ML 10X10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55150-0154-10 | EUGIA US LLC | 10 | 50MG/ML | SOLUTION |
PACKAGE FILES
Generic Name
ACYCLOVIR SODIUM
Substance Name
ACYCLOVIR SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAVENOUS
Application Number
ANDA203701
Description
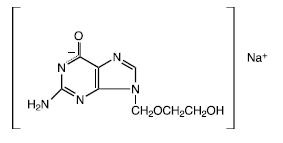
DESCRIPTION Acyclovir Sodium Injection is a synthetic nucleoside analog, active against herpes viruses. It is a sterile, aqueous solution for intravenous infusion, containing 50 mg acyclovir per mL in Water for Injection, USP. The concentration is equivalent to 54.9 mg of acyclovir sodium per mL in Water for Injection, USP. The sodium content is approximately 5.1 mg/mL. The pH range of the solution is 10.85 to 11.50. Further dilution of Acyclovir Sodium Injection in an appropriate intravenous solution must be performed before infusion (see DOSAGE AND ADMINISTRATION, Administration ). The chemical name of acyclovir sodium is 9-[(2-Hydroxyethoxy)methyl] guanine, and has the following structural formula: Acyclovir USP is a white to off-white, crystalline powder. Acyclovir sodium is the sodium salt of acyclovir, which is formed in situ , with the molecular formula C 8 H 10 N 5 NaO 3 and a molecular weight of 247.19. The maximum solubility in water at 25°C exceeds 100 mg/mL. At physiologic pH, acyclovir sodium exists as the unionized form with a molecular weight of 225 and a maximum solubility in water at 37°C of 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25. Acyclovir Sodium Chemical Structure
How Supplied
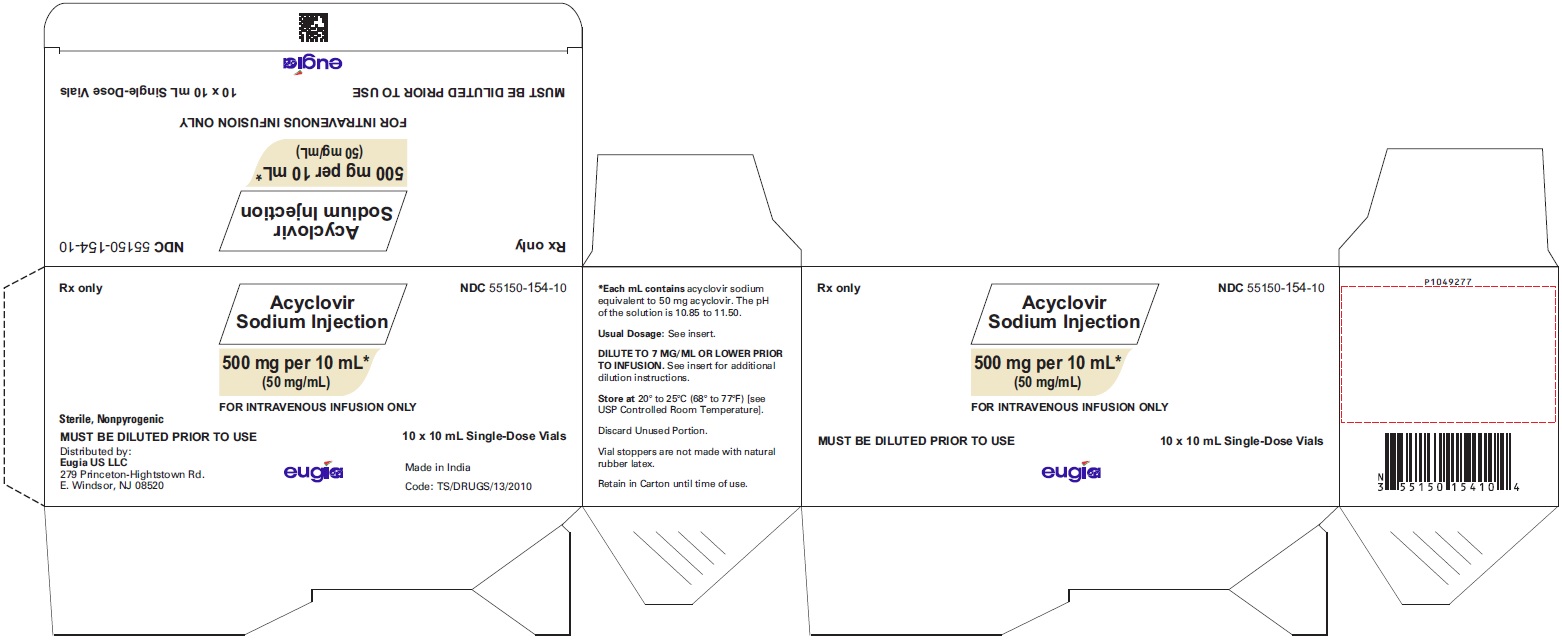
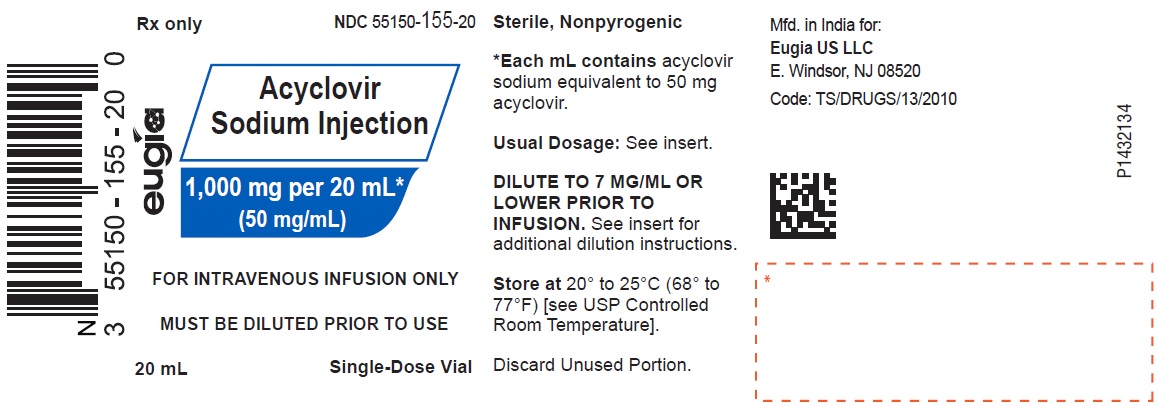
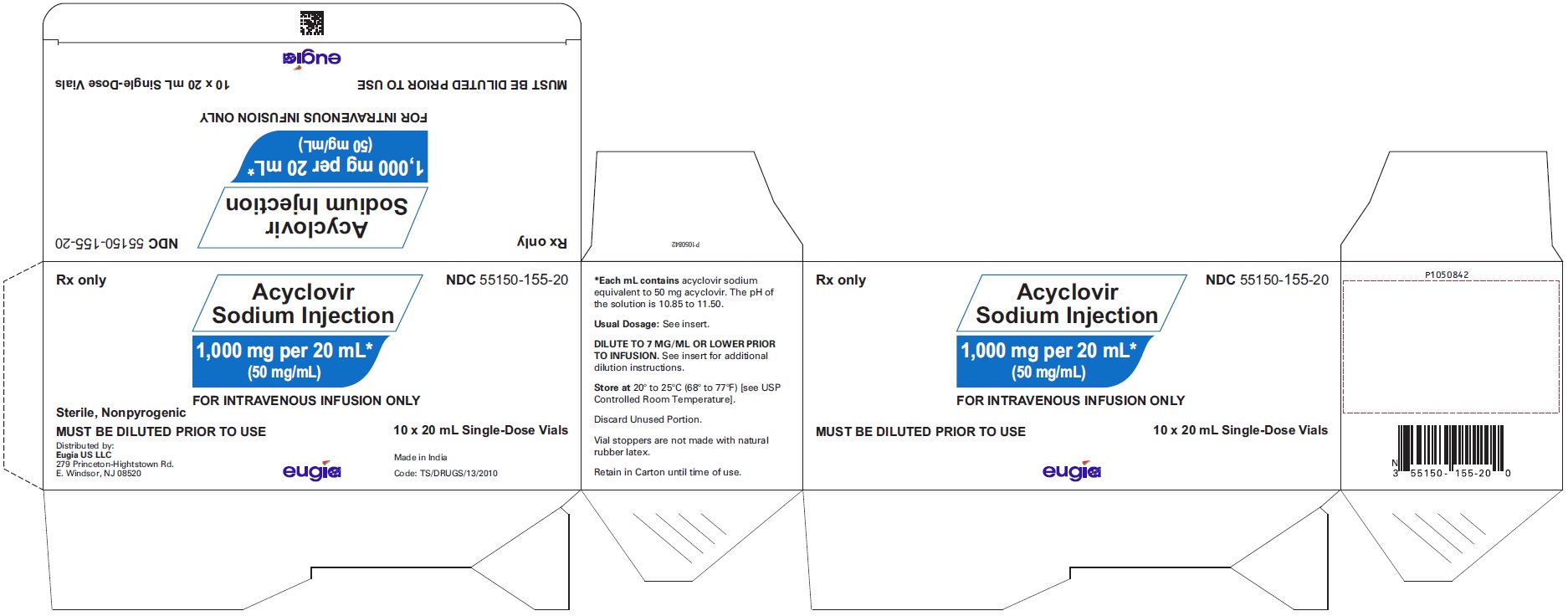
HOW SUPPLIED Acyclovir Sodium Injection is a clear, colorless solution and is available as: Acyclovir Sodium Injection equivalent to acyclovir, 50 mg/mL in 10 mL glass vials, in a Carton of 10 NDC 55150-154-10 Acyclovir Sodium Injection equivalent to acyclovir, 50 mg/mL in 20 mL glass vials, in a Carton of 10 NDC 55150-155-20 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Discard unused portion. Vial stoppers are not made with natural rubber latex. Retain in Carton until time of use. Sterile, Nonpyrogenic Distributed by: Eugia US LLC 279 Princeton-Hightstown Rd. E. Windsor, NJ 08520 Manufactured by: Eugia Pharma Specialities Limited Hyderabad - 500032 India Revised: March 2023
Indications & Usage
INDICATIONS AND USAGE Herpes Simplex Infections in Immunocompromised Patients Acyclovir Sodium Injection is indicated for the treatment of initial and recurrent mucosal and cutaneous herpes simplex (HSV-1 and HSV-2) in immunocompromised patients. Initial Episodes of Herpes Genitalis Acyclovir Sodium Injection is indicated for the treatment of severe initial clinical episodes of herpes genitalis in immuno-competent patients. Herpes Simplex Encephalitis Acyclovir Sodium Injection is indicated for the treatment of herpes simplex encephalitis. Neonatal Herpes Simplex Virus Infection Acyclovir Sodium Injection is indicated for the treatment of neonatal herpes infections. Varicella-Zoster Infections in Immunocompromised Patients Acyclovir Sodium Injection is indicated for the treatment of varicella-zoster (shingles) infections in immunocompromised patients.
Dosage and Administration
DOSAGE AND ADMINISTRATION CAUTION - RAPID OR BOLUS INTRAVENOUS INJECTION MUST BE AVOIDED (see WARNINGS and PRECAUTIONS ). INTRAMUSCULAR OR SUBCUTANEOUS INJECTION MUST BE AVOIDED (see WARNINGS ). Therapy should be initiated as early as possible following onset of signs and symptoms of herpes infections. A maximum dose equivalent to 20 mg/kg every 8 hours should not be exceeded for any patient. Dosage HERPES SIMPLEX INFECTIONS MUCOSAL AND CUTANEOUS HERPES SIMPLEX (HSV-1 AND HSV-2) INFECTIONS IN IMMUNOCOMPROMISED PATIENTS Adults and Adolescents (12 years of age and older) 5 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days. Pediatrics (Under 12 years of age) 10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days. SEVERE INITIAL CLINICAL EPISODES OF HERPES GENITALIS Adults and Adolescents (12 years of age and older) 5 mg/kg infused at a constant rate over 1 hour, every 8 hours for 5 days. HERPES SIMPLEX ENCEPHALITIS Adults and Adolescents (12 years of age and older) 10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days. Pediatrics (3 months to 12 years of age) 20 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days . Neonatal Herpes Simplex Virus Infections (Birth to 3 months) 10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 10 days. In neonatal herpes simplex infections, doses of 15 mg/kg or 20 mg/kg (infused at a constant rate over 1 hour every 8 hours) have been used; the safety and efficacy of these doses are not known. VARICELLA-ZOSTER INFECTIONS ZOSTER IN IMMUNOCOMPROMISED PATIENTS Adults and Adolescents (12 years of age and older) 10 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days. Pediatrics (Under 12 years of age) 20 mg/kg infused at a constant rate over 1 hour, every 8 hours for 7 days. Obese Patients Obese patients should be dosed at the recommended adult dose using Ideal Body Weight. PATIENTS WITH ACUTE OR CHRONIC RENAL IMPAIRMENT Refer to DOSAGE AND ADMINISTRATION section for recommended doses, and adjust the dosing interval as indicated in Table 5. Table 5: Dosage Adjustments for Patients with Renal Impairment Creatinine Clearance (mL/min/1.73 m 2 ) Percent of Recommended Dose Dosing Interval (hours) >50 100% 8 25 to 50 100% 12 10 to 25 100% 24 0 to 10 50% 24 Hemodialysis For patients who require dialysis, the mean plasma half-life of acyclovir during hemodialysis is approximately 5 hours. This results in a 60% decrease in plasma concentrations following a six-hour dialysis period. Therefore, the patient’s dosing schedule should be adjusted so that an additional dose is administered after each dialysis. Peritoneal Dialysis No supplemental dose appears to be necessary after adjustment of the dosing interval. Administration The calculated dose should be further diluted in an appropriate intravenous solution at a volume selected for administration during each 1 hour infusion. Infusion concentrations of approximately 7 mg/mL or lower are recommended. In clinical studies, the average 70 kg adult received between 60 and 150 mL of fluid per dose. Higher concentrations (e.g., 10 mg/mL) may produce phlebitis or inflammation at the injection site upon inadvertent extravasation. Standard, commercially available electrolyte and glucose solutions are suitable for intravenous administration; biologic or colloidal fluids (e.g., blood products, protein solutions, etc.) are not recommended. Once diluted for administration, each dose should be used within 24 hours. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
