Drug Catalog - Product Detail
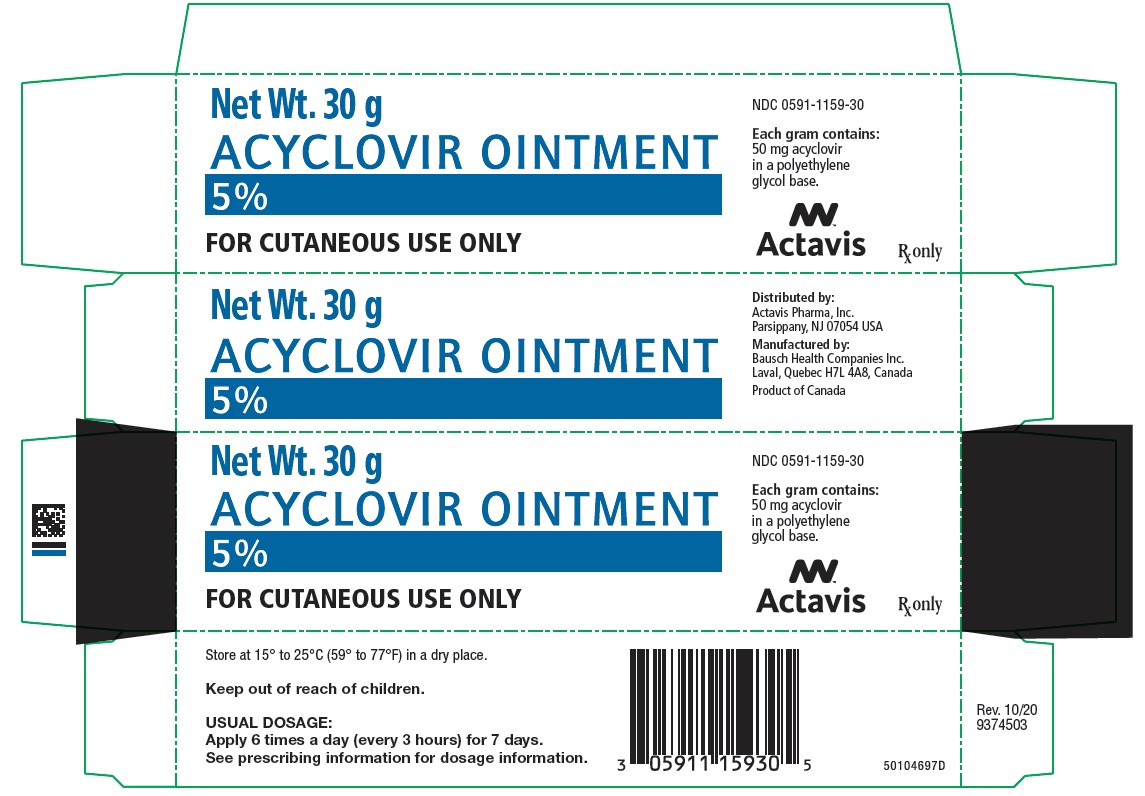
ACYCLOVIR OINTMENT OINT 0.05 30GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-1159-30 | ACTAVIS PHARMA | 30 | 5% | OINTMENT |
PACKAGE FILES

Generic Name
ACYCLOVIR
Substance Name
ACYCLOVIR
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
NDA018604
Description
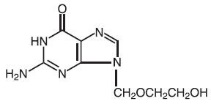
DESCRIPTION Acyclovir is a synthetic nucleoside analogue active against herpes viruses. Acyclovir Ointment 5% is a formulation for topical administration. Each gram of Acyclovir Ointment 5% contains 50 mg of acyclovir in a polyethylene glycol (PEG) base. Acyclovir is a white to off-white crystalline powder with the molecular formula C 8 H 11 N 5 O 3 and a molecular weight of 225.20. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir are 2.27 and 9.25. The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6 H -purin-6-one; it has the following structural formula: Chemical Structure
How Supplied
HOW SUPPLIED Each gram of Acyclovir Ointment 5% contains 50 mg acyclovir in a polyethylene glycol base. It is supplied as follows: 30 g tubes NDC 0591-1159-30 Store at 15° to 25°C (59° to 77°F) in a dry place.
Indications & Usage
INDICATIONS AND USAGE Acyclovir Ointment 5% is indicated in the management of initial genital herpes and in limited non-lifethreatening mucocutaneous HSV infections in immunocompromised patients.
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply sufficient quantity to adequately cover all lesions every 3 hours, 6 times per day for 7 days. The dose size per application will vary depending upon the total lesion area but should approximate a one-half inch ribbon of ointment per 4 square inches of surface area. A finger cot or rubber glove should be used when applying acyclovir to prevent autoinoculation of other body sites and transmission of infection to other persons. Therapy should be initiated as early as possible following onset of signs and symptoms.
