Drug Catalog - Product Detail
ACYCLOVIR OINTMENT 0.05 15GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0835-94 | AMNEAL PHARMACEUTICALS | 15 | 5% | OINTMENT |
PACKAGE FILES




Generic Name
ACYCLOVIR
Substance Name
ACYCLOVIR
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA204605
Description
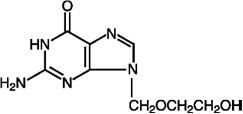
DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram of acyclovir ointment, USP 5% contains 50 mg of acyclovir, USP in a polyethylene glycol (PEG) base. Acyclovir, USP is a white, crystalline powder with the molecular formula C 8 H 11 N 5 O 3 and a molecular weight of 225. The maximum solubility in water at 37°C is 2.5 mg/mL. The pka’s of acyclovir, USP are 2.27 and 9.25. The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6 H -purin-6-one; it has the following structural formula: VIROLOGY Mechanism of Antiviral Action: Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro , acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the viral TK. Antiviral Activities: The quantitative relationship between the in vitro susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (IC 50 ), vary greatly depending upon a number of factors. Using plaque-reduction assays, the IC 50 against herpes simplex virus isolates ranges from 0.02 to 13.5 mcg/mL for HSV-1 and from 0.01 to 9.9 mcg/mL for HSV-2. The IC 50 for acyclovir against most laboratory strains and clinical isolates of VZV ranges from 0.12 to 10.8 mcg/mL. Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean IC 50 of 1.35 mcg/mL. Drug Resistance: Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV and VZV with reduced susceptibility to acyclovir have been recovered from immunocompromised patients, especially with advanced HIV infection. While most of the acyclovir-resistant mutants isolated thus far from immunocompromised patients have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults. The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy. eae4daa9-figure-01
How Supplied
HOW SUPPLIED Acyclovir Ointment, USP 5% is supplied as a white color ointment. Each gram of acyclovir ointment, USP 5% contains 50 mg acyclovir, USP in a polyethylene glycol base. It is supplied as follows: 15-g tubes (NDC 65162-835-94) 30-g tubes (NDC 65162-835-96) Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Store in a dry place. Distributed by: Amneal Pharmaceuticals Bridgewater, NJ 08807 Rev. 02-2016-01
Indications & Usage
INDICATIONS AND USAGE Acyclovir ointment 5% is indicated in the management of initial genital herpes and in limited non-life-threatening mucocutaneous Herpes simplex virus infections in immunocompromised patients.
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply sufficient quantity to adequately cover all lesions every 3 hours, 6 times per day for 7 days. The dose size per application will vary depending upon the total lesion area but should approximate a one-half inch ribbon of ointment per 4 square inches of surface area. A finger cot or rubber glove should be used when applying acyclovir ointment 5% to prevent autoinoculation of other body sites and transmission of infection to other persons. Therapy should be initiated as early as possible following onset of signs and symptoms.
