Drug Catalog - Product Detail
Acitretin 17.5mg Capsule 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1751-08 | AMNEAL PHARMACEUTICALS | 30 | 17.5MG | CAPSULE |
PACKAGE FILES







Generic Name
ACITRETIN
Substance Name
ACITRETIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202552
Description
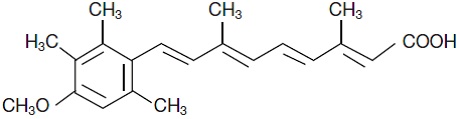
DESCRIPTION Acitretin, a retinoid, is available in 10 mg, 17.5 mg, 22.5 mg, and 25 mg gelatin capsules for oral administration. Chemically, acitretin is all-trans-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid. It is a metabolite of etretinate and is related to both retinoic acid and retinol (vitamin A). It is a yellow to greenish-yellow powder with a molecular weight of 326.44. The structural formula is: Each capsule contains acitretin, microcrystalline cellulose, maltodextrin, sodium ascorbate, gelatin, black imprinting ink (the solid components are shellac glaze, propylene glycol and iron oxide black). Gelatin capsule shells contain gelatin, red ferric oxide (10 mg, 22.5 mg and 25 mg only), yellow ferric oxide (17.5 mg and 25 mg only), sodium lauryl sulfate, and titanium dioxide (10 mg, 17.5 mg and 25 mg only). USP dissolution test is pending. Chemical Sructure of Acitretin
How Supplied
HOW SUPPLIED Acitretin Capsules USP, 10 mg are supplied as white to off-white body and a brown cap, imprinted in black “IX” on the capsule cap and “667” on the capsule body. They are available as follows: Bottles of 30: NDC 0115-1750-08 Acitretin Capsules USP, 17.5 mg are supplied as yellow to light yellow body and cap, imprinted in black “IX” on the capsule cap and “668” on the capsule body. They are available as follows: Bottles of 30: NDC 0115-1751-08 Acitretin Capsules USP, 22.5 mg are supplied as brown body and cap, imprinted in black with “IX” on the capsule cap and “698” on the capsule body. They are available as follows: Bottles of 30: NDC 0115-1752-08 Acitretin Capsules USP, 25 mg are supplied as yellow to light yellow body and a brown cap, imprinted in black “IX” on the capsule cap and “669” on the capsule body. They are available as follows: Bottles of 30: NDC 0115-1753-08 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. Avoid exposure to high temperatures and humidity after the bottle is opened.
Indications & Usage
INDICATIONS AND USAGE Acitretin capsules are indicated for the treatment of severe psoriasis in adults. Because of significant adverse effects associated with its use, acitretin capsules should be prescribed only by those knowledgeable in the systemic use of retinoids. In females of reproductive potential, acitretin capsules should be reserved for non-pregnant patients who are unresponsive to other therapies or whose clinical condition contraindicates the use of other treatments (see boxed CONTRAINDICATIONS AND WARNINGS — acitretin capsules can cause severe birth defects). Most patients experience relapse of psoriasis after discontinuing therapy. Subsequent courses, when clinically indicated, have produced efficacy results similar to the initial course of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION There is intersubject variation in the pharmacokinetics, clinical efficacy, and incidence of side effects with acitretin. A number of the more common side effects are dose-related. Individualization of dosage is required to achieve sufficient therapeutic response while minimizing side effects. Therapy with acitretin should be initiated at 25 mg per day to 50 mg per day, given as a single dose with the main meal. Maintenance doses of 25 mg per day to 50 mg per day may be given dependent upon an individual patient’s response to initial treatment. Relapses may be treated as outlined for initial therapy. When acitretin is used with phototherapy, the prescriber should decrease the phototherapy dose, dependent on the patient’s individual response (see PRECAUTIONS: General ). Females who have taken TEGISON (etretinate) must continue to follow the contraceptive recommendations for TEGISON. TEGISON is no longer marketed in the US; for information, call Amneal Pharmaceuticals at 1-877-835-5472. Information for Pharmacists Acitretin must only be dispensed in no more than a monthly supply. An Acitretin Medication Guide must be given to the patient each time acitretin is dispensed, as required by law.
