Drug Catalog - Product Detail
ACEBUTOLOL HCL CP 400MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0670-10 | AMNEAL PHARMACEUTICALS | 100 | 400MG | CAPSULE |
PACKAGE FILES


Generic Name
ACEBUTOLOL HYDROCHLORIDE
Substance Name
ACEBUTOLOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075047
Description
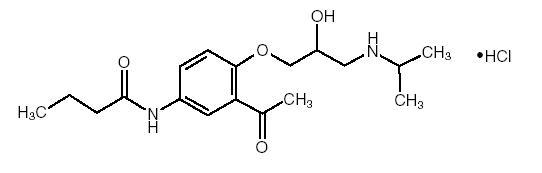
DESCRIPTION Acebutolol HCl, USP is a selective, hydrophilic beta-adrenoreceptor blocking agent with mild intrinsic sympathomimetic activity for use in treating patients with hypertension and ventricular arrhythmias. It is marketed in capsule form for oral administration. Acebutolol HCl capsules, USP are provided in two dosage strengths which contain 200 mg or 400 mg of acebutolol as the hydrochloride salt. The inactive ingredients present are D&C Red 28, D&C Yellow 10, FD&C Blue 1, FD&C Red 40, gelatin, maize starch, povidone, stearic acid and titanium dioxide. Acebutolol HCl, USP has the following structural formula: C 18 H 28 N 2 O 4 •HCl M.W. 372.89 Acebutolol HCl, USP is a white or slightly off-white powder freely soluble in water, and less soluble in alcohol. Chemically it is defined as the hydrochloride salt of (±) N-[3-Acetyl-4-[2-hydroxy-3-[(1-methylethyl) amino]propoxy]phenyl] butanamide. f1931f84-figure-01
How Supplied
HOW SUPPLIED Acebutolol hydrochloride capsules, USP are available as follows: 200 mg: Hard gelatin capsules with bright orange opaque body printed radially “669” with black ink and lavender opaque cap printed radially “Amneal” with black ink. Bottles of 100 NDC 65162-669-10 Bottles of 500 NDC 65162-669-50 400 mg: Hard gelatin capsules with bright orange opaque body printed radially “670” with black ink and lavender opaque cap printed radially “Amneal” with black ink. Bottles of 30 NDC 65162-670-03 Bottles of 100 NDC 65162-670-10 Bottles of 500 NDC 65162-670-50 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. Keep tightly closed. Dispense in a tight, light-resistant container. Distributed by: Amneal Pharmaceuticals Bridgewater, NJ 08807 Rev. 01-2016-02
Indications & Usage
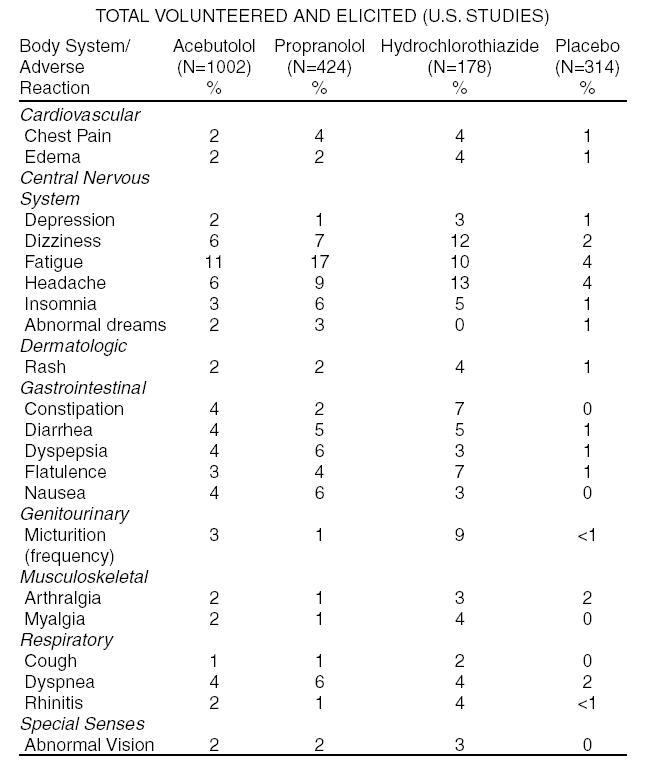
INDICATIONS AND USAGE Hypertension Acebutolol hydrochloride capsules, USP are indicated for the management of hypertension in adults. It may be used alone or in combination with other antihypertensive agents, especially thiazide-type diuretics. Ventricular Arrhythmias Acebutolol hydrochloride capsules, USP are indicated in the management of ventricular premature beats; it reduces the total number of premature beats, as well as the number of paired and multiform ventricular ectopic beats, and R-on-T beats.
Dosage and Administration
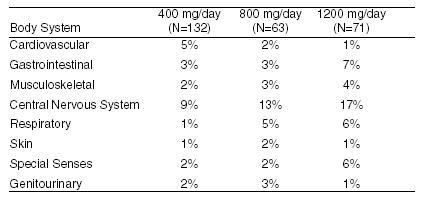
DOSAGE AND ADMINISTRATION Hypertension The initial dosage of acebutolol in uncomplicated mild-to-moderate hypertension is 400 mg. This can be given as a single daily dose, but in occasional patients twice daily dosing may be required for adequate 24-hour blood-pressure control. An optimal response is usually achieved with dosages of 400 to 800 mg per day, although some patients have been maintained on as little as 200 mg per day. Patients with more severe hypertension or who have demonstrated inadequate control may respond to a total of 1200 mg daily (administered b.i.d.), or to the addition of a second antihypertensive agent. Beta-1 selectivity diminishes as dosage is increased. Ventricular Arrhythmia The usual initial dose of acebutolol is 400 mg daily given as 200 mg b.i.d. Dosage should be increased gradually until an optimal clinical response is obtained, generally at 600 to 1200 mg per day. If treatment is to be discontinued, the dosage should be reduced gradually over a period of about two weeks. Use in Older Patients Older patients have an approximately 2-fold increase in bioavailability and may require lower maintenance doses. Doses above 800 mg/day should be avoided in the elderly.
