Drug Catalog - Product Detail
ACAMPROSATE CALCIUM TB 333MG 180
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-6333-80 | MYLAN | 180 | 333MG | TABLET |
PACKAGE FILES

Generic Name
ACAMPROSATE CALCIUM ENTERIC-COATED
Substance Name
ACAMPROSATE CALCIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200142
Description
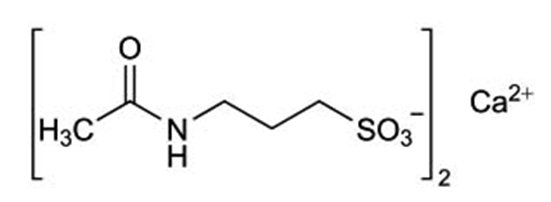
11 DESCRIPTION Acamprosate calcium is supplied in an enteric-coated tablet for oral administration. Acamprosate calcium is a synthetic compound with a chemical structure similar to that of the endogenous amino acid homotaurine, which is a structural analogue of the amino acid neurotransmitter γ-aminobutyric acid and the amino acid neuromodulator taurine. Its chemical name is 3-(Acetylamino)-1-propanesulfonic acid calcium salt (2:1). Its chemical formula is C 10 H 20 N 2 O 8 S 2 Ca and molecular weight is 400.48. Its structural formula is: Acamprosate calcium, USP is a white odorless crystalline powder. It is freely soluble in water, and practically insoluble in absolute ethanol and dichloromethane. Each acamprosate calcium delayed-release tablet contains acamprosate calcium 333 mg, equivalent to 300 mg of acamprosate. Inactive ingredients in acamprosate calcium delayed-release tablets include: colloidal silicon dioxide, glyceryl dibehenate, hypromellose, magnesium stearate, methacrylic acid copolymer type A, microcrystalline cellulose, propylene glycol and talc. In addition, the black imprinting ink for the tablets contains ammonium hydroxide, black iron oxide, propylene glycol and shellac glaze. Sulfites were used in the synthesis of the drug substance and traces of residual sulfites may be present in the drug product. Acamprosate Calcium Structural Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Acamprosate Calcium Delayed-Release Tablets are available containing 333 mg of acamprosate calcium, USP (equivalent to 300 mg of acamprosate). The 333 mg tablets are white, enteric-coated, round, unscored tablets imprinted with M over AC in black ink on one side of the tablet and plain on the other side. They are available as follows: NDC 0378-6333-80 bottles of 180 tablets Storage and Handling: Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Indications & Usage
1 INDICATIONS AND USAGE Acamprosate calcium delayed-release tablets are indicated for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation. Treatment with acamprosate calcium delayed-release tablets should be part of a comprehensive management program that includes psychosocial support. The efficacy of acamprosate calcium delayed-release tablets in promoting abstinence has not been demonstrated in subjects who have not undergone detoxification and not achieved alcohol abstinence prior to beginning acamprosate calcium delayed-release tablets treatment. The efficacy of acamprosate calcium delayed-release tablets in promoting abstinence from alcohol in polysubstance abusers has not been adequately assessed. • Acamprosate calcium delayed-release tablets are indicated for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation ( 1 , 14 ). • Treatment with acamprosate calcium delayed-release tablets should be part of a comprehensive management program that includes psychosocial support ( 1 ).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The recommended dose of acamprosate calcium delayed-release tablets is two 333 mg tablets (each dose should total 666 mg) taken three times daily. A lower dose may be effective in some patients. Although dosing may be done without regard to meals, dosing with meals was employed during clinical trials and is suggested in those patients who regularly eat three meals daily. Treatment with acamprosate calcium delayed-release tablets should be initiated as soon as possible after the period of alcohol withdrawal, when the patient has achieved abstinence, and should be maintained if the patient relapses. Acamprosate calcium delayed-release tablets should be used as part of a comprehensive psychosocial treatment program. • Recommended dose: 666 mg (two 333 mg tablets) taken three times daily ( 2 ). • Dose reduction to one 333 mg tablet taken three times daily for patients with moderate renal impairment (creatinine clearance 30-50 mL/min) ( 2.1 ). • Acamprosate calcium delayed-release tablets are contraindicated in patients with severe renal impairment (creatinine clearance of ≤ 30 mL/min) ( 2.1 , 4.2 , 5.1 , 8.6 , 12.3 ). 2.1 Dosage in Renal Impairment For patients with moderate renal impairment (creatinine clearance of 30-50 mL/min), a starting dose of one 333 mg tablet taken three times daily is recommended. Acamprosate calcium delayed-release tablets are contraindicated in patients with severe renal impairment (creatinine clearance of ≤ 30 mL/min) [ see Contraindications (4.2) , Warnings and Precautions (5.1) , Use in Specific Populations (8.6) , and Clinical Pharmacology (12.3) ] .
